Eteplirsen
 | |
| Clinical data | |
|---|---|
| Trade names | Exondys 51 |
| Routes of administration | Intravenous infusion |
| ATC code | None |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 1173755-55-9 |
| ChemSpider | 34983391 |
| UNII |
AIW6036FAS |
| Chemical and physical data | |
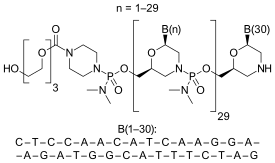
| Formula | C364H569N177O122P30 |
| Molar mass | 10305.738 |
| 3D model (Jmol) | Interactive image |
| |
| |
Eteplirsen, also called AVI-4658, is a drug designed for treatment, but not a cure, of some mutations that cause Duchenne muscular dystrophy (DMD), a genetic degenerative muscle disease. Eteplirsen is a product of Sarepta Therapeutics Inc. Eteplirsen only targets mutations in a region implicated in 13% of DMD cases.[1] After a controversial debate surrounding the efficacy of the drug, Eteplirsen received accelerated approval from the US Food and Drug administration in late 2016.[2][3] A year's worth of treatment with Epteplirsen is expected to cost approximately $300,000 dollars.[4]
Mechanism of action
Duchenne muscular dystrophy is caused when a mutation in the DMD gene changes the DMD RNA so that it no longer codes for functional dystrophin protein, usually due to a mutation that alters the reading frame of the RNA downstream of the mutation. If an exon with an appropriate number of bases lies near the mutation, by removing that exon the downstream reading frame can be corrected and production of partially functional dystrophin can be restored. This is the general strategy used for designing exon-skipping oligos for DMD; as there are 79 exons in the longest splice form of the dystrophin transcript, many different oligos are needed to address the range of mutations present in the population of people with DMD.
Eteplirsen is a morpholino antisense oligomer which triggers excision of exon 51 during pre-mRNA splicing of the dystrophin RNA transcript. Skipping exon 51 changes the downstream reading frame of dystrophin;[5] giving eteplirsen to a healthy person would result in production of dystrophin mRNA which would not code for functional dystrophin protein but, for DMD patients with particular frameshifting mutations, giving eteplirsen can restore the reading frame of the dystrophin mRNA and result in production of functional (although modified by having an internal deletion consisting of both the patient's original defect, as well as the therapeutically skipped exon) dystrophin.[6] Eteplirsen is given by intravenous infusion for systemic treatment of DMD.
Exon skipping is induced by eteplirsen, a charge-neutral, phosphorodiamidate morpholino oligomer (PMO) that selectively binds to exon of dystrophin pre-mRNA, restoring the open reading frame and enabling production of functional, but truncated, dystrophin.[7] The uncharged nature of the PMO helps make it resistant to biological degradation.[8] This truncated dystrophin protein produced by eteplirsen causes a less severe form of dystrophinopathy, much like Becker muscular dystrophy. PMO technology to treat other genotypes amenable to exon skipping to potentially treat an estimated 70 to 80% of all DMD patients with dystrophin gene deletion. Eteplirsen's proposed mechanism of action is increasing the production of muscle protein. By increasing the quantity of an abnormal, but potentially functional, dystrophin protein, the objective is to slow or prevent the progression of DMD.[7][9]
Clinical studies
Several clinical trials have been conducted to test eteplirsen, one in the UK involving local injection to the foot,[10][11] one in the UK involving systemic injection at low doses[12][1] and one in the USA at higher systemic doses[13] that progressed to a rollover extension study.[14][9] In the phase II study of 12 boys, dystrophin production was increased in 72% of the participants. It is questioned whether increasing the dose — which is feasible due to the lower toxicity of eteplirsen compared to drisapersen — would benefit the non-responders and whether this could result in any increased side effects. A phase III study has begun in the USA.[15]
In 2011, in a UK study eteplirsen (AVI-4658) was given to 19 children with Duchenne muscular dystrophy; researchers found that higher doses of the drug led to an increase in dystrophin. Researchers believe that drugs which are designed to make the body “skip over” mutations in this way could be used to treat approximately 83% of Duchenne muscular dystrophy cases. Eteplirsen only targets mutations in a region implicated in 13% of cases. This study demonstrated the potential of this approach for increasing the levels of dystrophin in the short term. The trial’s principal aim was to work out the appropriate dosages of the drug, therefore the drug’s safety profile and effects will need to be confirmed in larger, longer-term studies, particularly as patients would need to take it for the rest of their lives (or until a better treatment is available).[1]
A similar drug was also in clinical trials, drisapersen, which is a 2'-O-methyl phosphorothioate antisense oligo that, like eteplirsen, triggers skipping of dystrophin exon 51. In January 2016, the FDA rejected drisapersen (Kyndrisa) for lack of efficacy, effectively shifting focus to eteplirsen.[16]
Current status
Eterplirsen has received accelerated approval from the US FDA.[2]
Both eteplirsen and the similar drug drisapersen filed New Drug Application (NDA) for review with the US Food and Drug Administration (FDA).[17] The Prescription Drug User Fee Act (PDUFA) goal dates for these were December 27, 2015 for drisapersen and February 26, 2016 for eteplirsen. Following FDA rejection of drisapersen, the agency announced a three-month time extension for its review of eteplirsen. The agency was scheduled to make a decision on approval of eteplirsen by May 26, 2016. FDA's review of the drug (as per their briefing documents from January as well as April) questioned the efficacy of drug --- some of FDA's analysis contained questionable comparisons and inaccuracies, according to multiple letters to FDA from medical experts. On April 25, 2016, the Advisory Committee Panel voted against approval;[18] the negative vote was influenced by FDA's review as well very strict wording of the voting questions. In June 2016, FDA requested for additional data from Sarepta, to confirm findings of dystrophin production by Eteplirsen. After much deliberation and internal debate within FDA which required involvement of the FDA Commissioner, Eterplirsen received accelerated approval on September 19, 2016.
Pharmacokinetic (PK) properties and potential side effects
On January 22, 2016 the FDA Briefing Document containing information about eteplirsen (ND 206488) was submitted to the Peripheral and Central Nervous System Advisory Committee Meeting. The most common treatment for DMD is glucocorticoid use, which do not sufficiently ameliorate symptoms or address the underlying genetic mutation and lack of functional dystrophin. Part of the document included the following information pertaining to the pharmacokinetic properties of eteplirsen:[7]
- Clinical safety data shows that there has been no adverse effects from treatment with Eteplirsen based off the doses administered in several trials.
- In general, dose-proportionality and linearity in PK properties may be concluded following weekly doses of 0.5~20 mg/kg in Phase 1 dose-ranging study and 30 and 50 mg/kg in efficacy trials. There was insignificant drug accumulation following weekly dosing across this dose range of 0.5~50 mg/kg
- Eteplirsen is not metabolized by hepatic microsomes and was not a potent inducer or inhibitor of the major human CYP enzymes, and was not a substrate, nor did it have any major inhibitory potential for any of the key human drug transporters at the concentration range given in clinical trials. Based on these findings, it is expected to have a low potential for drug-drug interactions (DDI) in humans.
- Eteplirsen was found to be metabolically stable in vitro with no evidence of metabolism or metabolite formation.
- Most of the metabolism of eteplirsen is done through the kidneys.
- Following single or multiple IV infusion, the peak plasma concentrations (Cmax) of eteplirsen occurred near the end of infusion and plasma concentration-time profiles of eteplirsen were generally similar and showed multi-phasic decline; the majority of drug elimination occurred within 24 hours.
- Plasma protein binding of eteplirsen in human is relatively low, ranging 6.1~16.5% and is independent of concentration studied.
Nature and sequence of oligo and target
Eteplirsen is a morpholino phosphorodiamidate antisense oligomer.
CTCCAACATCAAGGAAGATGGCATTTCTAG (sequence source: US FDA ETEPLIRSEN BRIEFING DOCUMENT NDA 206488[7]),
30-mer,
20% G,
43% CG,
Predicted Tm: 88.9 °C at 10 µM oligo.
Oligo complement CTAGAAATGCCATCTTCCTTGATGTTGGAG
DMD-001 Exon 51, ENST00000357033.8 in Ensembl.org, RNA target site marked. Given that the target site is within an exon, this is likely blocking binding of an exonic splice enhancer protein and so altering splicing by interfering with splice regulation. CTCCTACTCAGACTGTTACTCTGGTGACACAACCTGTGGTTACTAAGGAAACTGCCATCT CCAAA[CTAGAAATGCCATCTTCCTTGATGTTGGAG]GTACCTGCTCTGGCAGATTTCAACC GGGCTTGGACAGAACTTACCGACTGGCTTTCTCTGCTTGATCAAGTTATAAAATCACAGA GGGTGATGGTGGGTGACCTTGAGGATATCAACGAGATGATCATCAAGCAGAAG
References
- 1 2 3 Cirak, Sebahattin; Arechavala-Gomeza, Virginia; Guglieri, Michela; Feng, Lucy; Torelli, Silvia; Anthony, Karen; Abbs, Stephen; Garralda, Maria Elena; Bourke, John; Wells, Dominic J; Dickson, George; Wood, Matthew JA; Wilton, Steve D; Straub, Volker; Kole, Ryszard; Shrewsbury, Stephen B; Sewry, Caroline; Morgan, Jennifer E; Bushby, Kate; Muntoni, Francesco (2011). "Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study". The Lancet. 378 (9791): 595–605. doi:10.1016/S0140-6736(11)60756-3. PMC 3156980
 . PMID 21784508. Lay summary – NHS Choices (July 25, 2011).
. PMID 21784508. Lay summary – NHS Choices (July 25, 2011). - 1 2 "FDA grants accelerated approval to first drug for Duchenne muscular dystrophy". Press Announcements. U.S. Food & Drug Administration. September 19, 2016. Retrieved September 19, 2016.
- ↑ "Railroading at the FDA" (PDF). Nature Biotechnology. 34 (11): 1078. November 2016. Retrieved 29 November 2016.
- ↑ Kounang, Nadia (4 October 2016). "The families that fought for controversial new drug". CNN. Retrieved 29 November 2016.
- ↑ Anthony, Karen; Feng, Lucy; Arechavala-Gomeza, Virginia; Guglieri, Michela; Straub, Volker; Bushby, Katherine; Cirak, Sebahattin; Morgan, Jennifer; Muntoni, Francesco (17 Oct 2012). "Exon Skipping Quantification by qRT-PCR in Duchenne Muscular Dystrophy Patients Treated with the Antisense Oligomer Eteplirsen". Hum Gene Ther Methods. 23 (5): 336–45. doi:10.1089/hgtb.2012.117. PMID 23075107.
- ↑ Moulton, HM; Moulton, JD (17 Feb 2010). "Morpholinos and Their Peptide Conjugates: Therapeutic Promise and Challenge for Duchenne Muscular Dystrophy". Biochim Biophys Acta. 1798 (12): 2296–303. doi:10.1016/j.bbamem.2010.02.012. PMID 20170628.
- 1 2 3 4 http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystemDrugsAdvisoryCommittee/UCM497063.pdf
- ↑ http://www.discoverymedicine.com/Ryszard-Kole/2012/07/26/targeting-mrna-splicing-as-a-potential-treatment-for-duchenne-muscular-dystrophy
- 1 2 Mendell, Jerry; Rodino-Klapac, Louise R; Sahenk, Zarife; Roush, Kandice; Bird, Loren; Lowes, Linda P; Alfano, Lindsay; Gomez, Ann Maria; Lewis, Sarah; Kota, Janaiah; Malik, Vinod; Shontz, Kim; Walker, Christopher M; Flanigan, Kevin M; Kean, John R; Allen, Hugh D; Shilling, Chris; Melia, Kathleen R; Sazani, Peter; Saoud, Jay B; Kaye, Edward M; Kaye, Edward M. (2013). "Eteplirsen for the treatment of duchenne muscular dystrophy". Ann. Neurol. 74 (5): n/a. doi:10.1002/ana.23982.
- ↑ Gary Roper, Manager Clinical Research Governance Organisation, Imperial College London. "Safety and Efficacy Study of Antisense Oligonucleotides in Duchenne Muscular Dystrophy". ClinicalTrials.gov. US Government, NIH. Retrieved 30 October 2012.
- ↑ Kinali, M; Arechavala-Gomeza, V; Feng, L; Cirak, S; Hunt, D; Adkin, C; Guglieri, M; Ashton, E; Abbs, S; Nihoyannopoulos, P; Garralda, ME; Rutherford, M; McCulley, C; Popplewell, L; Graham, IR; Dickson, G; Wood, MJ; Wells, DJ; Wilton, SD; Kole, R; Straub, V; Bushby, K; Sewry, C; Morgan, JE; Muntoni, F (25 Aug 2009). "Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: A single-blind, placebo-controlled, dose-escalation, proof-of-concept study". Lancet Neurol. 8 (10): 918–28. doi:10.1016/S1474-4422(09)70211-X. PMC 2755039
 . PMID 19713152.
. PMID 19713152. - ↑ Professor Francesco Muntoni, University College of London Institute of Child Health. "Dose-Ranging Study of AVI-4658 to Induce Dystrophin Expression in Selected Duchenne Muscular Dystrophy (DMD) Patients". ClinicalTrials.gov. US Government, NIH. Retrieved 30 October 2012.
- ↑ Sarepta Therapeutics. "Efficacy Study of AVI-4658 to Induce Dystrophin Expression in Selected Duchenne Muscular Dystrophy Patients". ClinicalTrials.gov. US Government, NIH. Retrieved 30 October 2012.
- ↑ Sarepta Therapeutics. "Efficacy, Safety, and Tolerability Rollover Study of Eteplirsen in Subjects With Duchenne Muscular Dystrophy". ClinicalTrials.gov. US Government, NIH. Retrieved 30 October 2012.
- ↑ Sarepta Therapeutics. "Confirmatory Study of Eteplirsen in DMD Patients (PROMOVI)". ClinicalTrials.gov. US Government, NIH. Retrieved 3 October 2014.
- ↑ FDA rejects BioMarin's muscle wasting drug; Sarepta drug in focus. Jan 2016
- ↑ "FDA Accepts Sarepta's NDA for Eteplirsen". Rare Disease Report.
- ↑ http://www.nytimes.com/2016/04/26/business/muscular-dystrophy-drug-fda-sarepta-eteplirsen.html