Epidermal growth factor
| View/Edit Human | View/Edit Mouse |
Epidermal growth factor (EGF) is a growth factor that stimulates cell growth, proliferation, and differentiation by binding to its receptor EGFR. Human EGF is a 6045-Da protein[3] with 53 amino acid residues and three intramolecular disulfide bonds.[4]
EGF was originally described independently as a secreted peptide found in the submaxillary glands of mice and in human urine. EGF has since been found in many human tissues including submandibular gland, parotid gland.[5] Initially, human EGF was known as urogastrone.[6]
Function
EGF [binding to EGFR] results in cellular proliferation, differentiation, and survival.[7]
Salivary EGF, which seems also regulated by dietary inorganic iodine, also plays an important physiological role in the maintenance of oro-esophageal and gastric tissue integrity. The biological effects of salivary EGF include healing of oral and gastroesophageal ulcers, inhibition of gastric acid secretion, stimulation of DNA synthesis as well as mucosal protection from intraluminal injurious factors such as gastric acid, bile acids, pepsin, and trypsin and to physical, chemical and bacterial agents.[5]
Biological sources
Epidermal growth factor can be found in urine, saliva, milk, and plasma.[8] The production of epidermal growth factor has been found to be stimulated by testosterone.
Mechanism
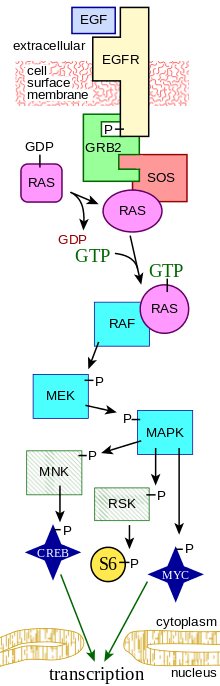
EGF acts by binding with high affinity to epidermal growth factor receptor (EGFR) on the cell surface. This stimulates ligand-induced dimerization,[9] activating the intrinsic protein-tyrosine kinase activity of the receptor (see the second diagram). The tyrosine kinase activity, in turn, initiates a signal transduction cascade that results in a variety of biochemical changes within the cell – a rise in intracellular calcium levels, increased glycolysis and protein synthesis, and increases in the expression of certain genes including the gene for EGFR – that ultimately lead to DNA synthesis and cell proliferation.[10]
EGF-family / EGF-like domain
EGF is the founding member of the EGF-family of proteins. Members of this protein family have highly similar structural and functional characteristics. Besides EGF itself other family members include:[11]
- Heparin-binding EGF-like growth factor (HB-EGF)
- transforming growth factor-α (TGF-α)
- Amphiregulin (AR)
- Epiregulin (EPR)
- Epigen
- Betacellulin (BTC)
- neuregulin-1 (NRG1)
- neuregulin-2 (NRG2)
- neuregulin-3 (NRG3)
- neuregulin-4 (NRG4).
All family members contain one or more repeats of the conserved amino acid sequence:
Where X represents any amino acid.[11]
This sequence contains 6 cysteine residues that form three intramolecular disulfide bonds. Disulfide bond formation generates three structural loops that are essential for high-affinity binding between members of the EGF-family and their cell-surface receptors.[12]
Interactions
Epidermal growth factor has been shown to interact with epidermal growth factor receptor.[13][14]
Medical uses
Recombinant human epidermal growth factor, sold under the brand name Heberprot-P, is used to treat diabetic foot ulcers. It can be given by injection into the wound site,[15] or may be used topically.[16] Tentative evidence shows improved wound healing.[17] Safety has been poorly studied.[17]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Harris RC, Chung E, Coffey RJ (March 2003). "EGF receptor ligands". Experimental Cell Research. 284 (1): 2–13. doi:10.1016/S0014-4827(02)00105-2. PMID 12648462.
- ↑ Carpenter G, Cohen S (May 1990). "Epidermal growth factor". The Journal of Biological Chemistry. 265 (14): 7709–12. PMID 2186024.
- 1 2 Venturi S, Venturi M (2009). "Iodine in evolution of salivary glands and in oral health". Nutrition and Health. 20 (2): 119–134. doi:10.1177/026010600902000204. PMID 19835108.
- ↑ Hollenberg, MD; Gregory, H (May 1980). "Epidermal growth factor-urogastrone: biological activity and receptor binding of derivatives.". Molecular Pharmacology. 17 (3): 314–20. PMID 6248761.
- ↑ Herbst RS (2004). "Review of epidermal growth factor receptor biology". International Journal of Radiation Oncology, Biology, Physics. 59 (2 Suppl): 21–6. doi:10.1016/j.ijrobp.2003.11.041. PMID 15142631.
- ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease. St. Louis, Mo: Elsevier Saunders. ISBN 0-7216-0187-1.
- ↑ Dawson JP, Berger MB, Lin CC, Schlessinger J, Lemmon MA, Ferguson KM (2005). "Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface". Mol. Cell. Biol. 25 (17): 7734–42. doi:10.1128/MCB.25.17.7734-7742.2005. PMC 1190273
 . PMID 16107719.
. PMID 16107719. - ↑ Fallon JH, Seroogy KB, Loughlin SE, Morrison RS, Bradshaw RA, Knaver DJ, Cunningham DD (June 1984). "Epidermal growth factor immunoreactive material in the central nervous system: location and development". Science. 224 (4653): 1107–9. doi:10.1126/science.6144184. PMID 6144184.
- 1 2 Dreux AC, Lamb DJ, Modjtahedi H, Ferns GA (May 2006). "The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis". Atherosclerosis. 186 (1): 38–53. doi:10.1016/j.atherosclerosis.2005.06.038. PMID 16076471.
- ↑ Harris RC, Chung E, Coffey RJ (2003). "EGF receptor ligands". Exp. Cell. Res. 284 (1): 2–13. doi:10.1016/S0014-4827(02)00105-2. PMID 12648462.
- ↑ Stortelers C, Souriau C, van Liempt E, van de Poll ML, van Zoelen EJ (July 2002). "Role of the N-terminus of epidermal growth factor in ErbB-2/ErbB-3 binding studied by phage display". Biochemistry. 41 (27): 8732–41. doi:10.1021/bi025878c. PMID 12093292.
- ↑ Wong L, Deb TB, Thompson SA, Wells A, Johnson GR (March 1999). "A differential requirement for the COOH-terminal region of the epidermal growth factor (EGF) receptor in amphiregulin and EGF mitogenic signaling". J. Biol. Chem. 274 (13): 8900–9. doi:10.1074/jbc.274.13.8900. PMID 10085134.
- ↑ Berlanga, J.; Fernández, J. I.; López, E.; López, P. A.; del Río, A.; Valenzuela, C.; Baldomero, J.; Muzio, V.; Raíces, M.; Silva, R.; Acevedo, B. E.; Herrera, L. (2013). "Heberprot-P: a novel product for treating advanced diabetic foot ulcer". MEDICC Review. 15 (1): 11–15. PMID 23396236.
- ↑ Yang, S; Geng, Z; Ma, K; Sun, X; Fu, X (June 2016). "Efficacy of Topical Recombinant Human Epidermal Growth Factor for Treatment of Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis.". The international journal of lower extremity wounds. 15 (2): 120–5. PMID 27151755.
- 1 2 Martí-Carvajal, AJ; Gluud, C; Nicola, S; Simancas-Racines, D; Reveiz, L; Oliva, P; Cedeño-Taborda, J (28 October 2015). "Growth factors for treating diabetic foot ulcers.". The Cochrane database of systematic reviews (10): CD008548. PMID 26509249.
Further reading
- Boonstra J, Rijken P, Humbel B, Cremers F, Verkleij A, van Bergen en Henegouwen P (1995). "The epidermal growth factor". Cell Biol. Int. 19 (5): 413–30. doi:10.1006/cbir.1995.1086. PMID 7640657.
- Dvorak B (2004). "Epidermal growth factor and necrotizing enterocolitis". Clinics in perinatology. 31 (1): 183–92. doi:10.1016/j.clp.2004.03.015. PMID 15183666.
- Howell WM (2004). "Epidermal growth factor gene polymorphism and development of cutaneous melanoma". J. Invest. Dermatol. 123 (4): xx–xxi. doi:10.1111/j.0022-202X.2004.23308.x. PMID 15373802.
External links
| Wikimedia Commons has media related to Epidermal growth factor, EGF. |
- Shaanxi Zhongbang Pharma-Tech Co., Ltd.-Supply of Epidermal Growth Factor
- EGF at the Human Protein Reference Database.
- Epidermal growth factor at the US National Library of Medicine Medical Subject Headings (MeSH)
- EGF model in BioModels database