Dental plaque
Dental plaque is a biofilm or mass of bacteria that grows on surfaces within the mouth. It is a sticky colorless deposit at first, but when it forms tartar it is brown or pale yellow that is commonly found between the teeth, front of teeth, behind teeth, on chewing surface, along the gumline, or below the gumline cervical margins.[1] Dental plaque is also known as microbial plaque, oral biofilm, dental biofilm, dental plaque biofilm or bacterial plaque biofilm.[1] While plaque is commonly associated with oral diseases such as cavities and periodontal diseases (gum diseases), its formation is a normal process that cannot be prevented.
Dental plaque can give rise to dental caries (tooth decay) – the localised destruction of the tissues of the tooth by acid produced from the bacterial degradation of fermentable sugar – and periodontal problems such as gingivitis and periodontitis.[2] Its progression and build up is what leads to oral problems, hence it is important to disrupt the mass of bacteria and remove it daily.[3] Plaque control and removal is achieved with correct tooth brushing and use of interdental aids such as dental floss and interdental brushes.[1]
Removal of dental biofilm is important as it may become acidic causing demineralization of the teeth (also known as caries) or harden into calculus (dental) (also known as tartar).[4] Calculus cannot be removed through toothbrushing or with interdental aids and can only be removed through professional cleaning.[2] Therefore, removal of the dental biofilm will prevent the development of caries and gum diseases.[3]
Plaque formation
Dental plaque is a biofilm that attaches to tooth surfaces, restorations and prosthetic appliances (including dentures, bridge (dentistry)) if left undisturbed. Understanding the formation, composition and characteristics of plaque helps in its control.[5] An acquired pellicle is a layer of saliva that is composed of mainly glycoproteins and forms shortly after cleaning of the teeth or exposure of new teeth.[6] Bacteria then attach to the pellicle layer, forms micro-colonies, and matures on the tooth which can result in oral diseases. The following table provides a more detailed (six step) explanation of biofilm formation:

Components of plaque
In the oral cavity, there are different types of bacteria that normally present in the mouth. These bacteria and leukocytes, neutrophils, macrophages, and lymphocytes are part of the normal oral cavity and contribute to the individual's health.[1] Approximately 80–90% of the weight of plaque is water. While 70% of the dry weight is bacteria, the remaining 30% consists of polysaccharides and glycoproteins.[7] Streptococcus mutans and anaerobes are the initial colonisers of the tooth surface, and play a major role in the establishment of the early biofilm community.[8]
Bacteria
The microorganisms that form the biofilm are mainly Streptococcus mutans and anaerobes, with the composition varying by location in the mouth. Examples of such anaerobes include fusobacterium and actinobacteria.[1] These microorganisms present in dental plaque are all naturally present in the oral cavity, and are normally harmless. However, failure to remove plaque by regular tooth brushing means that they are allowed to build up in a thick layer and cause dental disease. Those microorganisms nearest the tooth surface ferment dietary sucrose; it is in this state that they start to produce acids.
The bacterial equilibrium position varies at different stages of formation. Below is a summary of the bacteria that may be present during the phases of plaque maturation.
- Early biofilm: primarily gram-positive cocci
- Older biofilm (3–4 days): increased numbers of filaments and fusiforms
- 4–9 days undisturbed: more complex flora with rods, filamentous forms
- 7–14 days: vibrios, spirochetes, more gram-negative organisms[9]
Supragingival biofilm
This is dental plaque that forms above the gums. This is the first kind of plaque that forms after the brushing of the teeth. It commonly forms in between the teeth, in the pits and grooves of the teeth and along the gums. It is made up of mostly aerobic bacteria, meaning these bacteria need oxygen to survive. As plaque remains on the tooth for a longer period of time, anaerobic bacteria begins to form in this plaque.[5]
Subgingival biofilm
Subgingival biofilm is plaque that is located under the gums. It occurs after the formation of the supragingival biofilm by a downward growth of the bacteria from above the gums to below. This plaque is mostly made up of anaerobic bacteria, meaning that these bacteria will only survive if there is no oxygen. As this plaque attaches in a pocket under the gums, no oxygen in the mouth can reach this bacteria meaning it will thrive if not removed.[9]
The extracellular matrix contains proteins, long chain polysaccharides and lipids.
The most common reasons for ecosystem disruption are the ecological factors discussed in the environment section. The bacteria that exhibits the most fit plasticity for the change in environment dominates the given environment. Often, this could lead to opportunistic pathogens that lead to dental caries and periodontal disease. Pathogens that have the potential to cause dental caries flourish in acidic environments. Pathogenic bacteria that have the potential to cause periodontal disease flourish in a slightly alkaline environment.[10]
Environment
The mouth is a warm and moist environment that is able to support the growth and development of dental plaque.[11] The main ecological factors that contribute to plaque formation are pH, saliva, temperature and redox reactions.[12][13] The normal pH range of saliva is between 6 and 7 and plaque biofilm is known to flourish in a pH between 6.7 and 8.3.[14][15] This indicates that the natural environment of the mouth provided by saliva is ideal for the growth of bacteria in the dental plaque. Saliva acts as a buffer, which helps to maintain the pH in the mouth between 6 and 7.[12] In addition to acting as a buffer, saliva and gingival crevicular fluid contain primary nutrients including amino acids, proteins and glycoproteins. This feeds the bacteria involved in plaque formation. The host diet plays only a minor role in providing nutrients for the resident microflora.[11] The normal temperature of the mouth ranges between 35–36 °C and a two degree (°C) change has been shown to drastically shift the dominant species in the plaque.[13] Redox reactions are carried out by aerobic bacteria. This keeps the oxygen levels in the mouth at a semi-stable homeostatic condition, which allows the bacteria to survive.[13]
Consequence of plaque build up
Gingivitis

Gingivitis is a common result of plaque build up around the gingival tissues. The bacteria found in the biofilm elicit a host response resulting in localized inflammation of the tissue.[16] This is characterized by the cardinal signs of inflammation including a red, puffy appearance of the gums and bleeding due to brushing or flossing.[17] Gingivitis due to plaque can be reversible by removal of the cause, plaque. However if left for an extended period the inflammation may begin to affect the supporting tissues, in a progression referred to as periodontitis.[18] The gingivitis response is a protective mechanism, averting periodontitis in many cases.
Periodontitis

Periodontitis is an infection of the gums which leads to bone destruction around the teeth in the jaw. Periodontitis occurs after gingivitis has been established, but not all individuals who have gingivitis will get periodontitis.[19][20] Plaque accumulation is vital in the progression of periodontitis as the bacteria in plaque release enzymes which attack the bone and cause it to break down, and at the same time the osteoclasts in the bone breakdown the bone as a way to prevent further infection. This can be treated with strict oral hygiene such as tooth brushing and cleaning in between the teeth and as well as surgical debridement completed by a dental professional.[21]
Caries
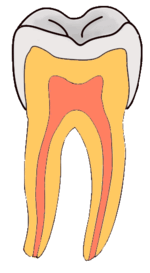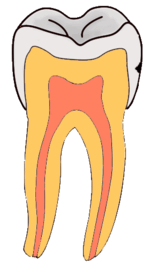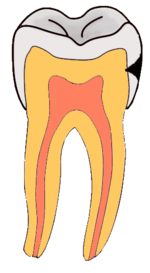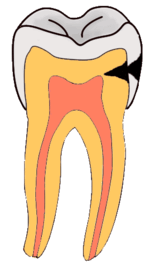
Dental caries is an infectious disease caused primarily by Streptococcus mutans, characterized by acid demineralization of the enamel, which can progress to further breakdown of the more organic, inner dental tissue.[1] Everybody is susceptible to caries but the probability of development depends on the patient’s individual disease indicators, risk factors and preventive factors. Factors that are considered high risk for developing carious lesions include:
- Low fluoride exposure
- Time, length, and frequency of sugar consumption
- Quality of tooth cleaning
- Fluctuations in salivary flow rates and composition
- Behavior of the individual
- Socioeconomic status of the individual
- Quality and composition of biofilms.[1]
Acids released from dental plaque lead to demineralization of the adjacent tooth surface, and consequently to dental caries. Saliva is also unable to penetrate the build-up of plaque and thus cannot act to neutralize the acid produced by the bacteria and remineralize the tooth surface.
Detection of plaque build up
Plaque detection is usually detected clinically by plaque disclosing agents. Disclosing agents contain dye which turns bright red to indicate plaque buildup.[1]
There are two main methods of detecting dental plaque in the oral cavity; through the application of a disclosing gel/tablet and/or visually through observation. It is important for an individual to be aware of what to look for when doing a self assessment for dental plaque. It is important to be aware that everyone has dental plaque, however there are varying levels of severity and consequences of not removing the dental plaque biolfilm.[1]
Plaque disclosing gel

Plaque disclosing products, also known as disclosants, make plaque clinically visible. Clean surfaces of the teeth do not absorb the disclosant, only rough surfaces. Plaque disclosing gels can be either completed at home or in the dental clinic. Before using these at home or in the dental clinic check with your general practitioners for any allergies for Iodine, food colouring or any other ingredients that may be present in these products. These gels provide a visual aid in assessing plaque biolfim presence and can also show the maturity of the dental plaque.
Disclosing tablets
Main article: Disclosing tablet

Disclosing tablets are similar to that of disclosing gels. However they are placed in the mouth and chewed on for approximately one minute. The remaining tablet or saliva is then spit out. Disclosing gels will show the presence of the plaque, but will often not show the level of maturity of the plaque. Disclosing tablets are often prescribed or given to patients with orthodontic appliances for use before and after toothbrushing to ensure optimal cleaning. These are also helpful educational tools for young children or patients that are struggling to remove dental plaque in certain areas.
Disclosing gels and tablets are useful for individuals of all ages in ensuring efficient dental plaque removal.
Visual or tactile detection of dental plaque
Dental biofilm forms on the tooth only minutes after brushing. It can be difficult to visualise dental plaque on the hard tissue surfaces, however a rough surface can be felt after eating or before toothbrushing if there is a thick deposit of dental plaque. It is often felt as a thick, fur-like deposit that may present as a yellow, tan or brown stain. They are commonly found on teeth or dental appliances such as orthodontic brackets and the tongue. The most common way dental plaque is assessed, is through dental assessment in the dental clinic where dental instruments are able to scrape up some plaque.
The most common areas where patients find plaque are between the teeth or along the cervical margins.
Plaque in dogs and cats
Plaque is extremely common in companion animals such as dogs or cats. However, the bacteria associated with canine and feline plaque appear to be different from that of humans.[22] [23] If untreated it can lead to more severe gum disease such as periodontitis, hence vets often recommend oral healthcare products for affected pets.
See also
References
- 1 2 3 4 5 6 7 8 9 Darby M L, Walsh M M. Dental Hygiene Theory and Practice. 2010.
- 1 2 Wolf H and Hassell T (2006). Color Atlas of Dental Hygiene, Thieme New York, 333 Seventh Avenue, New York, USA.
- 1 2 Verkaik M, Busscher H, Jager D, Slomp A, Abbas F, van der Mei H. "Efficacy of natural antimicrobials in toothpaste formulations against oral biofilms in vitro". Journal of Dentistry. 2011;39(3):218-224.
- ↑ Summitt J, R. J., Hilton T, Schwartz R. (2006). Fundamentals of Operative Dentistry. 4350 Chandler Drive, Hanover Park, Illinois.
- 1 2 Chetrus V and Ion I.R (2013). "Dental Plaque - Classification, Formation, and Identification." Int. J. Med. Detistry 3: 139-143.
- ↑ Kreth, Jens; Merritt, J.; Qi, F. (August 2009). "Bacterial and Host Interactions of Oral Streptococci". DNA and Cell Biology. Mary Ann Liebert, Inc. 28 (8): 397–403. doi:10.1089/dna.2009.0868. Retrieved 2012-08-22. (subscription required (help)).
- ↑ P D Marsh, D J Bradshaw. "Dental plaque as a biofilm." Journal of Industrial Microbiology. 1995. Volume 15. ك Number 3. Page 169.
- ↑ Kolenbrander P. "Oral Microbial Communities: Biofilms, Interactions, and Genetic Systems" 1. Annu Rev Microbiol. 2000;54(1):413–437.
- 1 2 Wilkins E. Clinical Practice of the Dental Hygienist. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009
- ↑ Garcia, F.; Hicks, M.J. (May 2008). "Maintaining the Integrity of the Enamel Surface: The role of dental biofilm, saliva, and preventative agents in the enamel demineralization and remineralization". Journal of the American Dental Association. American Dental Association. 139 (suppl. 2): 25S–34S. Retrieved 2012-08-22.
- 1 2 Marsh PD, Moter A, Devine DA. (2011). "Dental plaque biofilms: communities, conflict, and control". Periodontology 2000. 55(1), 16-35.
- 1 2 Marsh, P.D. (February 2003). "Are dental diseases examples of ecological catastrophes?". Microbiology. Society for General Microbiology. 143 (2): 279–294. doi:10.1099/mic.0.26082-0. Retrieved 2012-08-22.
- 1 2 3 Marsh, P.D.; Devine, D.A. (February 2011). "How is the development of dental biofilms influenced by the host?". Journal of Clinical Periodontology. John Wiley & Sons. 38 (s11): 28–35. doi:10.1111/j.1600-051X.2010.01673.x. Retrieved 2012-08-22.
- ↑ Humphrey, S. P., & Williamson, R. T. (2001). A review of saliva: normal composition, flow, and function. The Journal of prosthetic dentistry, 85(2), 162-169.
- ↑ McDermid A, McKee A, Marsh P. "Effect of Environmental pH on Enzyme Activity and Growth of Bacteroides gingivalis W50". Infection and Immunity. 1998;56(5):1096-100.
- ↑ The American Academy of Periodontology. Proceedings of the World Workshop in Clinical Periodontics. Chicago:The American Academy of Periodontology; 1989:I/23-I/24.
- ↑ Parakrama Chandrasoma, Clive R. Taylor (c. 2005). "Part A. General Pathology, Section II. The Host Response to Injury, Chapter 3. The Acute Inflammatory Response, sub-section Cardinal Clinical Signs". Concise Pathology (3rd edition (Computer file) ed.). New York, N.Y.: McGraw-Hill. ISBN 0-8385-1499-5. OCLC 150148447. Retrieved 2008-11-05.
- ↑ Noble SL. Clinical Textbook of Dental Hygiene and Therapy, 2nd ed. West Sussex, Wiley-Blackwell; 2012. p. 96-97.
- ↑ Noble SL. Clinical Textbook of Dental Hygiene and Therapy, 2nd ed. West Sussex, Wiley-Blackwell; 2012. p. 111.
- ↑ Rateitschak KH, Rateitschak EM, Wolf HF, Hassell TM. Color Atlas of Periodontology, New York, Thieme Inc; 1985. p.55
- ↑ Tonetti M S, Eickholz P, Loos B G, Papapanou P, van der Velden U, Armitage G, Bouchard P, Deinzer R, Dietrich T, Hughes F, Kocher T, Lang N P, Lopez R, Needleman I, Newton T, Nibali L, Pretzl B, Ramseier C, Sanz-Sanchez I, Schlagenhauf U, Suvan J E, Fabrikant E, Fundak A. "Principles in Prevention of Periodontal Diseases". Journal of Clinical Periodontology 2015, http://onlinelibrary.wiley.com/doi/10.1111/jcpe.12368/full?justLoggedIn=true&rememberMePresent=false.
- ↑ The feline oral microbiome: A provisional 16S rRNA gene based taxonomy with full-length reference sequences. Floyd E. Dewhirsta, Erin A. Klein, Marie-Louise Bennett, Julie M. Croft, Stephen J. Harris, Zoe V. Marshall-Jones. Journal of Veterinary Microbiology, Volume 175, Issues 2–4, 25 February 2015, pp 294–303
- ↑ Dewhirst FE, Klein EA, Thompson EC, Blanton JM, Chen T, Milella L, et al. (2012) The Canine Oral Microbiome. PLoS ONE 7(4): e36067.