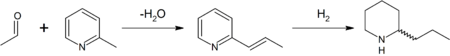Coniine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2S)-2-propylpiperidine | |
| Identifiers | |
| 3238-60-6 (R/S) 5985-99-9 (R) 458-88-8 (S) | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:28322 |
| ChEMBL | ChEMBL2287063 |
| ChemSpider | 389878 |
| ECHA InfoCard | 100.006.621 |
| KEGG | C06523 |
| PubChem | 441072 |
| UNII | C479P32L2D |
| |
| |
| Properties | |
| C8H17N | |
| Molar mass | 127.23 g·mol−1 |
| Melting point | −2 °C (28 °F; 271 K) |
| Boiling point | 166 to 167 °C (331 to 333 °F; 439 to 440 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Coniine is a poisonous alkaloid found in poison hemlock (Conium maculatum), the yellow pitcher plant (Sarracenia flava),[1] and fool's parsley (Aethusa cynapium)[2] and contributes to hemlock's fetid smell. It disrupts the peripheral nervous system and is toxic to humans and all classes of livestock; less than 0.1g is fatal to humans, with death caused by respiratory paralysis. Notably, Socrates was sentenced to death by drinking a mixture containing poison hemlock[3][4] in 399 BC.[5]
Coniine has two stereoisomers: (S)-(+)-coniine, and (R)-(−)-coniine, both of which are present in hemlock (see "Modern Chemical Studies", below). Coniine was first synthesized by Albert Ladenburg in 1886; it was the first of the alkaloids to be synthesized.
Natural origins
Poison hemlock
Poison hemlock (Conium maculatum) contains not only highly toxic amounts of coniine, but also trace amounts of four other similarly poisonous alkaloids. Ingesting less than a tenth of a gram of coniine can be fatal for adult humans; this is approximately six to eight hemlock leaves.[6] The seeds and roots are also toxic —more so than the leaves, in fact. While hemlock toxicity primarily results from consumption, poisoning can also result from inhalation, and from skin contact.[7]

The presence of hemlock on farmland is an issue for livestock farmers because animals will eat it if they are not well fed or the hemlock is mixed in with pasture grass. Farmers also need to be careful that the hay fed to animals does not contain hemlock.[8] Poison hemlock is most poisonous in the spring when the concentration of y-coniciene (the precursor to other toxins) is at its peak.[9][10]
Poison hemlock grows quite tall, reaching heights of up to twelve feet.[11] The stalk of hemlock is green with purple spots and completely lacks hair. A biennial plant, hemlock produces leaves at its base the first year but no flowers. In its second year it produces white flowers in umbrella shaped clusters.[12] Hemlock can be confused with the wild carrot plant; however, this plant has a hairy stem without purple markings, grows less than three feet tall, and does not have clustered flowers.[7] While the hemlock plant is native to Europe and the Mediterranean region,[13] it has spread to every other continent excluding Antarctica.[14]

Yellow pitcher plant
The yellow pitcher plant (Sarracenia flava) is a carnivorous plant found exclusively in the southeastern United States that produces coniine. The plant uses a mixture of sugar and coniine to simultaneously attract and poison insects, which then fall into a digestive tube. The naming of the plant arises from the shape of the opening to these tubes, which resembles a pitcher. The pitcher shape has a leaf above to prevent rain from diluting the digestive fluids deep in the tubes. These pitchers can grow up to three feet tall. In the spring the plant produces yellow flowers with five petals each.[15]
There are no reports online of human poisoning via the yellow pitcher plant, perhaps because only a small portion of the plant contains coniine, or because it does not contain enough to produce toxicity. It is also not as widespread as hemlock and therefore is less likely to be contacted by humans.
History
The history of coniine is understandably tied to the poison hemlock plant since it was not synthesizable until modern times. While the yellow pitcher plant also contains coniine, it has no traditional uses.
The most famous hemlock poisoning occurred in 399 B.C.E. when the philosopher Socrates drank a liquid infused with hemlock to carry out his death sentence (Socrates had been convicted of impiety toward the gods and corruption of the youth). In fact, hemlock juice was often used to execute criminals in ancient Greece.[16]
Hemlock has had a limited medical use throughout history. The Greeks used it not just as capital punishment, but also as an antispasmodic and treatment for arthritis.[17] Books from the 10th century attest to medical use by the Anglo-Saxons.[18] In the Middle Ages it was believed that hemlock could be used to cure rabies;[19] in later European times it came to be associated with witchcraft.[19][20] Native Americans used hemlock extract as arrow poison.[14]
Coniine is the poison used to kill Amyas Crale in Five Little Pigs (published in 1943), also known as Murder in Retrospect, one of Agatha Christie's Hercule Poirot mysteries.
Coniine was first isolated by Giesecke,[21] but the formula was suggested by Blyth[22] and definitely established by Hoffmann.[23]
Chemical properties
D-(S)-Coniine is a colorless alkaline liquid, with a penetrating odour and a burning taste; has D0° 0.8626 and D19° 0.8438, refractive index n23°D 1.4505, and is dextrorotatory, [α]19°D +15.7°. (See comments about the specific rotation below, under "Enantiomers"). L-(R)-Coniine has [α]21°D 15° and in other respects resembles its D-isomer, but the salts have slightly different melting points; the platinichloride has mp. 160 °C (Löffler and Friedrich report 175 °C), the aurichloride mp. 59 °C.[24][25]
Solubility
Coniine is slightly soluble (1 in 90) in cold water, less so in hot water, so that a clear cold solution becomes turbid when warmed. On the other hand, the base dissolves about 25% of water at room temperature. It mixes with alcohol in all proportions, is readily soluble in ether and most organic solvents. Coniine dissolves in carbon disulfide, forming a complex thiocarbamate.[26][27]
Crystallization
Coniine solidifies into a soft crystalline mass at −2 °C. It slowly oxidizes in the air. The salts crystallize well and are soluble in water or alcohol. The hydrochloride, B•HCl, crystallizes from water in rhombs, mp. 220 °C, [α]20°D +10.1°; the hydrobromide, in needles, mp. 211 °C, and the D-acid tartrate, B•C4H6O6•2 H2O, in rhombic crystals, mp. 54 °C. The platinichloride, (B•HCl)2•PtCl4•H2O, separates from concentrated solution as an oil, which solidifies to a mass of orange-yellow crystals, mp. 175 °C (dry). The aurichloride, B•HAuCl4, crystallizes on standing, mp. 77 °C. The picrate forms small yellow needles, mp. 75 °C, from hot water. The 2,4-dinitrobenzoyl- and 3,5-dinitrobenzoyl-derivates have mps. 139.0–139.5 °C and 108–9 °C respectively.[28] The precipitate afforded by potassium cadmium iodide solution is crystalline, mp. 118 °C, while that given by nicotine with this reagent is amorphous.
Color changes
It gives no coloration with sulfuric or nitric acid. Sodium nitroprusside gives a deep red color, which disappears on warming, but reappears on cooling, and is changed to blue or violet by aldehydes.[29]
Enantiomers
Naturally-occurring coniine is present in Conium maculatum as a mixture of the R-(−)- and S-(+)- enantiomers, although the S-enantiomer predominates.[30]
The stereochemical composition of "coniine" is a matter of some importance, since its two enantiomers do not have identical biological properties,[30] and many of the older pharmacological studies on this compound were undoubtedly carried out using the naturally-occurring isomeric mixture.
The common criterion for enantiomeric homogeneity is the specific rotation, [α]D. This value depends on such factors as temperature, solvent and concentration of the analyte, but it is also important to note that a salt such as the hydrochloride of a given enantiomer will not necessarily have the same specific rotation as the same enantiomer of the free base.
Modern values for the specific rotation of the enantiomers of coniine, and their hydrochloride salts are as follows:
S-(+)-coniine (which is identical to D-(+)-coniine, but the "D/L" and "S/R" systems of stereochemical nomenclature are not usually mixed together in the same enantiomer name) has [α]D = +8.4° (c = 4.0, in CHCl3)[31] These authors note that Ladenburg's value of +15°[32] is for a "neat" sample, i.e., one that is undiluted with any solvent.
A similarly high value of +16° for the [α]D of "coniine" is given, without explicit citation of the source, in The Merck Index.[33]
The value of +7.7° (c = 4.0, CHCl3) for synthetic S-(+)-coniine and -7.9° (c = 0.5, CHCl3) for synthetic R-(−)-coniine is given by other chemists.[34]
The hydrochloride salt of S-(+)-coniine has [α]D = +4.6° (c = 0.5, in methanol).[30]
The hydrochloride salt of R-(−)-coniine has [α]D = -5.2° (c = 0.5, in methanol).[30]
Many syntheses of coniine have been reported over the last 50 years; one example of a stereoselective synthesis is that of Enders and Tiebes,[34] who cite some of the earlier preparations.
Synthesis
In the original synthesis of this substance by Ladenburg in 1886,[35] he heated methylpyridinium iodide at 250 °C to obtain 2-methylpyridine (α-picoline). 2-Methylpyridine was reacted with paraldehyde in the presence of a base to 2-propenylpyridine in a Knoevenagel condensation. This intermediate was reduced with metallic sodium in ethanol to racemic (±) coniine (reduction by hydrogen gas is also possible). Enantiopure coniine was obtained by chiral resolution — fractional crystallisation of the diastereoisomers of the salt obtained with (+)-tartaric acid.
The initial reaction, however, gives a poor yield and was improved by interaction of the two reagents at 150 °C in sealed tubes to give methyl-2-picolylalkyne, which was then heated at 185 °C with hydrochloric acid for 10 hours, producing a mixture of 2-propenylpyridine and 2-chloropropylpyridine. This mixture was reduced to rac-coniine by sodium in ethanol.
{The final structure in the above reaction scheme is drawn as that of a single enantiomer, although the final reaction produces a racemic product.}
In 1907 the process was still further improved by reducing 2-(2'-hydroxypropyl)pyridine with phosphorus and hydroiodic acid at 125 °C and treating the product with zinc dust and water, then reducing the product with sodium in ethanol.[36][37][38][39][40][41][42][43][44][45][46]
{The final structure in the above reaction scheme is drawn as that of a single enantiomer, although the final reaction produces a racemic product.}
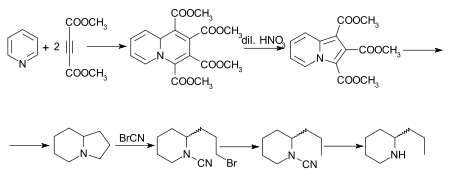
A number of other syntheses of coniine have been effected,[47][48][49][50][51] of which that of Diels and Alder is of special interest. The initial adduct of pyridine and dimethyl acetylenedicarboxylate is tetramethylquinolizine-1,2,3,4-tetracarboxylate, which on oxidation with dilute nitric acid is converted into trimethyl indolizine-tricarboxylate. This, on hydrolysis and decarboxylation, furnishes indolizine, the octahydro-derivate of which, also known as octahydropyrrocoline[52] is converted by the cyanogen bromide method successively into the bromocyanoamide, cyanoamide and rac.-coniine. A synthesis of the alkaloid, starting from indolizine (pyrrocoline) is described by Ochiai and Tsuda.[53]
The preparation of L-(R)-coniine by the reduction of β-coniceine (L-propenylpiperidine) by Löffler and Friedrich[25] is interesting as a means of passing from conhydrine to L-(R)-coniine. Hess and Eichel reported,[54] incorrectly,[55] that pelletierine was the aldehyde (β-2-piperidyl-propaldehyde) corresponding to coniine, and yielded rac-coniine when its hydrazone was heated with sodium ethoxide in ethanol at 156–170 °C. According to these authors, D-(S)-coniine is rendered almost optically inactive when heated with barium hydroxide and alcohol at 180–230 °C. Leithe[56] has shown by observation of the optical rotation of (+)-pipecolic acid (piperidine-2-carboxylic acid) and some of its derivatives under varying conditions,[57] that it must belong to the D-series of amino acids, and since (+)-conhydrine can be oxidised to (−)-pipecolic acid,[58] and transformed through β-coniceine into L-(R)-(−)-coniine,[25] it follows that (+)-coniine, (+)-2-methylpiperidine (α-pipecoline) and (+)-piperidine-2-carboxylic acid must all have similar spatial configurations.
Much of the early chemical research on coniine, up to ~1950, was reviewed by Marion.[59]
Pharmacology
Coniine paralyzes muscles by blocking the nicotinic receptor on the post-synaptic membrane of the neuromuscular junction causing a flaccid paralysis. This action is similar to that of curare. Symptoms of paralysis occur within a half-hour, and death may take several hours. As the central nervous system is not affected the person remains conscious and aware until respiratory paralysis results in cessation of breathing. The muscular paralysis is an ascending flaccid paralysis as the lower limbs are affected first. The person may have a hypoxic convulsion just prior to death but this is greatly disguised by the muscular paralysis and the person may just weakly shudder. The cause of death is lack of oxygen to the brain and heart as a consequence of respiratory paralysis. A poisoned person will recover if artificial ventilation (breathing) is maintained until the toxin is removed from the receptor.
Toxicology
The LD50 (mouse; administered i.v.) for R-(−)-coniine is ~7 mg/kg; for a racemic mixture it is ~8 mg/kg; for S-(+)-coniine it is ~12 mg/kg.[30]
References
- ↑ N. V. Mody; R. Henson; P. A. Hedin; U. Kokpol; D. H. Miles (1976). "Isolation of the insect paralyzing agent coniine from Sarracenia flava". Experientia. 32 (7): 829–830. doi:10.1007/BF02003710.
- ↑ Clapham, Tutin, & Warburg: Flora of the British Isles, 2nd edition, page 524
- ↑ James Warren (2001). "Socratic suicide". Journal of Hellenic Studies. 121: 91–106. doi:10.2307/631830. PMID 19681231.
- ↑ R. G. Frey (1978). "Did Socrates commit suicide?". Philosophy. 53 (203): 106–108. doi:10.1017/S0031819100016375.
- ↑
 Jackson, Henry (1911). "Socrates". In Chisholm, Hugh. Encyclopædia Britannica. 25 (11th ed.). Cambridge University Press. pp. 331–338.
Jackson, Henry (1911). "Socrates". In Chisholm, Hugh. Encyclopædia Britannica. 25 (11th ed.). Cambridge University Press. pp. 331–338. SOCRATES, son of the statuary Sophroniscus and of the midwife Phaenarete, was born at Athens, not earlier than 471 nor later than May or June 469 B.C. ... In 399, four years after the restoration and the amnesty, he was indicted as an offender against public morality. ... The accusation ran thus: “Socrates is guilty, firstly, of denying the gods recognized by the state and introducing new divinities, and, secondly, of corrupting the young.” ... Under ordinary circumstances the condemned criminal drank the cup of hemlock on the day after the trial; but in the case of Socrates the rule that during the absence of the sacred ship sent annually to Delos no one should be put to death caused an exceptional
- ↑ Conium maculatum
- 1 2 "Poison-hemlock". King County. Retrieved 3 May 2015.
- ↑ Peters, Amy; Bouska, Cassie. "Poison Hemlock". OSU Extension Service. Oregon State University. Retrieved 3 May 2015.
- ↑ Cheeke, Peter (31 Aug 1989). Toxicants of Plant Origin: Alkaloids, Volume 1 (1 ed.). Boca Raton, Florida: CRC Press. p. 118. ISBN 0849369908.
- ↑ "Poison Hemlock: Options for Control" (PDF). co.lincoln.wa.us. Lincoln County Noxious Weed Control Board. Retrieved 3 May 2015.
- ↑ "Poison Hemlock". pierecountryweedboard.wsu.edu. Pierce County Noxious Weed Control Board.
- ↑ "Poison Hemlock" (PDF). store.msuextension.org. Montana State University. Retrieved 3 May 2015.
- ↑ Vetter, J (September 2004). "Poison Hemlock (Conium maculatum L.)". Food Chem Toxicol (42): 1374–82. PMID 15234067.
- 1 2 Moser, L; Crisp, D. "Poison Hemlock" (PDF). San Francisco Peaks Weed Management. Retrieved 3 May 2015.
- ↑ Mackie, Robin. "Yellow Pitcher Plant or Trumpets". United States Department of Agriculture. United States Department of Agriculture Forest Service. Retrieved 3 May 2015.
- ↑ "The Suicide of Socrates". EyeWitness to History. Retrieved 3 May 2015.
- ↑ Tucker, Mitch. "Hemlock and Death of Socrates". Evolution of Hemlock. University of Oklahoma. Retrieved 3 May 2015.
- ↑ Grieve, M. (1971). A Modern Herbal (2nd ed.). Mineola, N.Y.: Dover Publications. p. 392. ISBN 0-486-22798-7. Retrieved 3 May 2015.
- 1 2 "Hemlock - Britain's Most Common "Witchy" Plant". White Dragon. Rowan. Retrieved 3 May 2015.
- ↑ Flying ointment
- ↑ Arch. Pharm., 1827, 20, 97.
- ↑ Annalen, 1849, 70, 73.
- ↑ Ber., 1881, 14, 705.
- ↑ Ahrens, Ber., 1902, 35, 1330
- 1 2 3 Löffler and Friedrich, Ber., 1909, 42, 107.
- ↑ Melzer, Arch. Pharm., 1898, 236, 701
- ↑ cf. Dilling, Pharm. J., 1909, [iv], 29, 34, 70, 102.
- ↑ Späth, Kuffner and Ensfellner, Ber., 1933, 66, 596.
- ↑ Gabutti, Chem. Soc. Abstr., 1906, [ii], 711.
- 1 2 3 4 5 Stephen T. Lee; Benedict T. Green; Kevin D. Welch; James A. Pfister; Kip E. Panter (2008). "Stereoselective potencies and relative toxicities of coniine enantiomers". Chemical Research in Toxicology. 21 (10): 2061–2064. doi:10.1021/tx800229w.
- ↑ Craig J. Cymerman; A. R. Pinder (1971). "Improved method resolution of coniine". Journal of Organic Chemistry. 36 (23): 3648–3649. doi:10.1021/jo00822a051.
- ↑ A. Ladenburg (1888) Justus Liebig's Ann. Chem. 247 1-98.
- ↑ The Merck Index, 15th Ed. (2013), p. 446, Monograph 2489, O'Neil: The Royal Society of Chemistry. Available online at: http://www.rsc.org/Merck-Index/monograph/mono1500002489
- 1 2 D. Enders and J. Tiebes (1993) Liebig's Ann. Chem. 173-177.
- ↑ Chem. Soc. Abstr., 1886, 19, 439, 2579.
- ↑ Ber., 1893, 26, 854
- ↑ Ber., 1894, 27, 853, 859, 3063
- ↑ Ber., 1896, 29, 2706
- ↑ Ber., 1901, 34, 3416
- ↑ Ber., 1906, 39, 2486
- ↑ Ber., 1907, 40, 3734
- ↑ cf. Wolffenstein, Ber., 1894, 27, 2611, 2615
- ↑ Ber., 1896, 29, 1956
- ↑ Simon, Bull. Soc. chim., 1894, [iii], 9, 949
- ↑ Landolt, Ber., 1894, 27, 1362
- ↑ Hess and Weltzien, Ber., 1920, 53, 139.
- ↑ Engler and Baur, Ber., 1891, 24, 2530
- ↑ Ber., 1894, 27, 1775
- ↑ Lautenschlager and Onsanger, Ber., 1918, 51, 602
- ↑ Koller, Monats., 1926, 47, 393
- ↑ Diels and Alder, Annalen, 1932, 498, 16.
- ↑ G. R. Clemo; G. R. Ramage (1932). "Octahydropyrrocoline". Journal of the Chemical Society: 2969–2973. doi:10.1039/JR9320002969.
- ↑ Ber., 1934, 67, 1011.
- ↑ Ber., 1917, 50, 1192, 1386.
- ↑ Pelletierine is now known to be 1-(2-piperidinyl)-2-propanone; see: The Merck Index, 15th Ed. (2013), p. 1314, Monograph 7181, O'Neil: The Royal Society of Chemistry. Available online at: http://www.rsc.org/Merck-Index/monograph/mono1500007181
- ↑ Ber., 1932, 65, 927.
- ↑ George William Clough (1918). "The relationship between the optical rotatory powers and the relative configurations of optically active compounds. The influence of certain inorganic haloids on the optical rotatory powers of α-hydroxy-acids, α-amino-acids, and their derivatives". Journal of the Chemical Society, Transactions. 113: 526–554. doi:10.1039/CT9181300526.
- ↑ Willstätter, Ber., 1901, 34, 3166.
- ↑ L. Marion (1950). In The Alkaloids, Vol. 1 (R. H. F. Manske and H. L. Holmes, Eds.), pp. 211-217, New York: Academic Press.