Cobatoxin
 Space-filling diagram of cobatoxin 1 | |
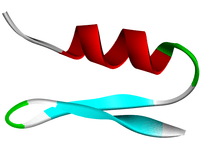 Ribbon diagram of cobatoxin 1 | |
| Identifiers | |
|---|---|
| 211688-17-4 | |
| Properties | |
| C156H245N51O44S6 | |
| Molar mass | 3,731.35 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cobatoxin is a toxin present in the venom of the scorpion Centruroides noxius. It blocks two potassium channel subtypes; voltage-gated and calcium-activated channels.
Etymology
Coba is a region in Mexico where the Centruroides noxius scorpion is found.
Sources
Cobatoxin 1 and 2 are both found in the venom of the Centruroides noxius scorpion.[1] [2]
Chemistry
Structure
Cobatoxin is a 32-residue toxin with 3 disulfide bridges, which are located on C1-C4, C2-C5 and C3- C6 (Cys3-Cys22, Cys8-Cys27, and Cys12-Cys29). The peptide backbone is folded according to an α/β scaffold, both α-helical and two-stranded β-sheet structures are present. Cobatoxin 1 has a rod-like shape due to an extended N-terminus.[1][2]
Family
Cobatoxin 1 and 2 both belong to the α-KTx family. The α-KTx family is part of the K+-channel-specific scorpion toxins (KTx), which consists of 3 families; α, β, and γ. These 3 families differ in structure.[3]
Target
Cobatoxin 1 and 2 both block the Kv1.1 K+-channels in mice and the Shaker B K+ channels in insects, which are voltage-dependent K+-channels (Selisko, 1998). Other voltage-dependent K+-channels that are blocked by Cobatoxin 1 are the Kv1.2 K+-channels in rats and Kv1.3 K+-channels in mice. Cobatoxin 1 also blocks the IKCa1 Ca2+-activated K+-channel.[2]
Mode of action
Cobatoxin 1 is a pore-blocking toxin. The interaction between cobatoxin 1 and the Kv1.2 channel is first esthablished by four salt bridges, which are formed between side chains of the four Kv1.2 α-subunits and amino acids residues of cobatoxin 1. This way a stable complex is formed, named the toxin-ring. Next, a tighter interaction is formed by a hydrophobic interaction between cobatoxin 1 and the α-subunit. Then the Lys21 side chain of cobatoxin 1 blocks the pore by entering the P-domain of the ion channel, which is the selectivity filter.[2]
Toxicity
The LD50 of cobatoxin 1 after intracerebroventricular injection in mice is 500±45 ng.[2]
References
- 1 2 Selisko B, Garcia C, Becerril B, Gómez-Lagunas F, Garay C, Possani LD (Jun 1998). "Cobatoxins 1 and 2 from Centruroides noxius Hoffmann constitute a subfamily of potassium-channel-blocking scorpion toxins". Eur J Biochem. 254 (3): 468–79. doi:10.1046/j.1432-1327.1998.2540468.x. PMID 9688256.
- 1 2 3 4 5 Jouirou B, Mosbah A, Visan V, et al. (Jan 2004). "Cobatoxin 1 from Centruroides noxius scorpion venom: chemical synthesis, three-dimensional structure in solution, pharmacology and docking on K+ channels". Biochem J. 377 (Pt 1): 37–49. doi:10.1042/BJ20030977. PMC 1223841
 . PMID 14498829.
. PMID 14498829. - ↑ Tytgat J, Chandy KG, Garcia ML, et al. (Nov 1999). "A unified nomenclature for short-chain peptides isolated from scorpion venoms: alpha-KTx molecular subfamilies". Trends Pharmacol Sci. 20 (11): 444–7. doi:10.1016/S0165-6147(99)01398-X. PMID 10542442.