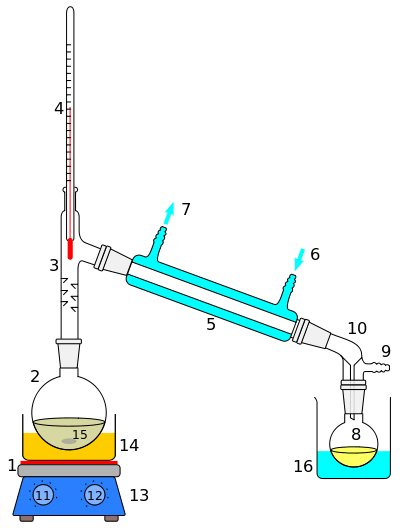
Destructive distillation
Destructive distillation is the chemical process involving the decomposition of feedstock by heating it to a high temperature; the term generally applies to processing of organic material in the absence of air or in the presence of limited amounts of oxygen or other reagents, catalysts, or solvents, such as steam or phenols. It is an application of pyrolysis. The process breaks up or 'cracks' large molecules. Coke, coal gas, gas carbon, coal tar, Buckminsterfullerene, ammonia liquor, and "coal oil" historically, are examples of commercial products of the destructive distillation of coal.
Destructive distillation of any particular inorganic feedstock produces only a small range of products as a rule, but destructive distillation of organic materials commonly produces very many compounds, often hundreds, though not all chemical products of any particular process are of commercial importance. The distilled molecules are generally smaller and more volatile than the feedstock molecules, but some reactions polymerise or condense small molecules into larger molecules, including heat-stable tarry substances and chars. Cracking into liquid and volatile compounds, and polymerisation or the formation of chars and solids may all occur in the same process, and any class of the products might be of commercial interest.
Currently the major industrial application of destructive distillation is to coal.[1][2]
Historically the process of destructive distillation and other forms of pyrolysis led to the discovery of many chemical compounds or elucidation of their structures before contemporary organic chemists had developed the processes to synthesise or specifically investigate the parent molecules. It was especially in early days that investigation of the products of destructive distillation, like those of other destructive processes, played parts in enabling chemists to deduce the chemical nature of many natural materials.[3] Well known examples include the deduction of the structures of pyranoses and furanoses.[4]
Process
The process of pyrolysis can be conducted in a distillation apparatus (retort) to form the volatile products for collection. The mass of the product will represent only a part of the mass of the feedstock, because much of the material remains as char and non-volatile tars. This is in contrast to simple combustion, where the fuel is oxidized; typically the combustion products of a hydrocarbon fuel amount to roughly four times the mass of the fuel consumed.
Destructive distillation and related processes are in effect the modern industrial versions of traditional charcoal burning crafts. As such they are of industrial significance in many regions, such as Scandinavia. The modern processes are sophisticated and require careful engineering to produce the most valuable possible products from the available feedstocks.[5][6]
Applications
- Destructive distillation of wood produces hundreds of compounds including tar, terpenes, turpentine and methanol together with a solid residue of charcoal.[7][8]
- Destructive distillation of a ton of coal can produce 700 kg of coke, 100 liters of liquor ammonia, 50 liters of coal tar and 400 m3 of coal gas.
- Destructive distillation is an increasingly promising method for recycling monomers derived from waste polymers.
- Destructive distillation of natural rubber resulted in the discovery of Isoprene which led to the creation of synthetic rubbers such as neoprene.[9]
See also
References
- ↑ Lunge, George. Coal-tar and ammonia Publisher: Gurney and Jackson,1887. May be downloaded from: https://archive.org/details/coaltarandammon00lunggoog
- ↑ Speight, James G. The Refinery of the Future. Publisher: William Andrew 2010 ISBN 978-0-8155-2041-2
- ↑ Schorlemmer, Carl, Smithells, Arthur. The rise and development of organic chemistry. Publisher: Macmillan 1894. May be downloaded from: https://archive.org/details/risedevelopmento00schorich
- ↑ I.L. Finar Organic Chemistry vol 1 ( 4th.ed.) Longmans 1963 plus I.L. Finar Organic Chemistry vol 2 ( 3rd.ed.) Longmans Green & Co. 1964 May be downlaoded from: https://archive.org/details/OrganicChemistryVol1 plus https://archive.org/details/OrganicChemistryVol2
- ↑ Bates, John S.; Distillation of hardwoods in Canada; Pub: Ottawa, F. A. Acland, 1922. May be downloaded from:
- ↑ Klar, Max; Rule, Alexander; The technology of wood distillation, with special reference to the methods of obtaining the intermediate and finished products from the primary distillate; Pub: London Chapman & Hall 1925. May be downloaded from:
- ↑ Loos, Hermann A.; A Study on Colophony Resin; Columbia University 1900. May be downloaded from:
- ↑ DUMESNY, P. & NOYER J.; Wood products, distillates and extracts; Pub: Scott, Greenwood & Son, 1908. May be downloaded from:
- ↑ C. Greville Williams On Isoprene and Caoutchine Proceedings of the Royal Society of London, Vol. 10, (1859 - 1860), pp. 516-519. URL: http://www.jstor.org/stable/111688.