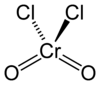
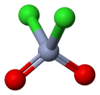
Chromyl chloride
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Chromium(VI) dichloride dioxide | |||
| Systematic IUPAC name
Dichlorodioxochromium | |||
| Other names
Chromic acid chloride Chromium oxychloride Chlorochromic anhydride Chromic oxychloride Chromium chloride oxide Chromium dioxide dichloride Chromium dioxychloride Chromium oxychloride | |||
| Identifiers | |||
| 14977-61-8 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:33038 | ||
| ChemSpider | 21106426 | ||
| ECHA InfoCard | 100.035.491 | ||
| EC Number | 239-056-8 | ||
| PubChem | 22150757 | ||
| RTECS number | GB5775000 | ||
| |||
| |||
| Properties | |||
| CrO2Cl2 | |||
| Molar mass | 154.9008 g/mol | ||
| Appearance | deep red fuming liquid | ||
| Odor | musty, burning, acrid[1] | ||
| Density | 1.911 g/mL, liquid | ||
| Melting point | -96.5 °C | ||
| Boiling point | 117 °C | ||
| Reacts[1] | |||
| Vapor pressure | 20 mmHg (20 °C)[1] | ||
| Hazards | |||
| Main hazards | carcinogen, reacts violently with water[1] | ||
| R-phrases | R49 R46 R8 R35 R43 R50/53 | ||
| S-phrases | S53 S45 S60 S61 | ||
| Flash point | noncombustible [1] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
none[1] | ||
| REL (Recommended) |
Ca TWA 0.001 mg Cr(VI)/m3[1] | ||
| IDLH (Immediate danger) |
N.D.[1] | ||
| Related compounds | |||
| Related compounds |
SO2Cl2; VOCl3; MoO2Cl2; WO2Cl2; CrO2F2 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Chromyl chloride is a chemical compound with the formula CrO2Cl2. This compound is an opaque dark blood-red liquid at room temperature and pressure. It is tetrahedral, somewhat like SO2Cl2. CrO2Cl2 is similar to the most commonly encountered chromium(VI) derivative chromate, [CrO4]2−; both are tetrahedral Cr(VI) compounds. However, they differ in both oxidizing power and in physical properties: one is a liquid and the other forms salts.
CrO2Cl2 is a neutral molecular species. This means that in the liquid and solid forms, the individual CrO2Cl2 entities interact predominantly via van der Waals bonding. Such weak bonding leads to low melting and boiling points, which is related to the fact that it is a distillable liquid.
The diminished oxidizing power of [CrO4]2− vs. CrO2Cl2 can be ascribed to its anionic nature, which diminishes its electron affinity. Also, chloride is a poorer pi-donor ligand than is oxide.
Preparation
Chromyl chloride can be prepared by mixing potassium chromate or potassium dichromate with sodium chloride and treating this mix with concentrated sulfuric acid, followed by gentle distillation. [2]
K2Cr2O7 + 4NaCl + 6H2SO4 → 2CrO2Cl2 + 2KHSO4 + 4NaHSO4 +3H2O
Chemical properties
CrO2Cl2 is highly electrophilic and an aggressive oxidizing agent, e.g. causing spontaneous combustion when dripped onto amorphous sulfur; can also oxidize toluene into benzaldehyde.[3] Its electrophilicity is demonstrated by its rapid hydrolysis to give chromic acid and hydrochloric acid:
- CrO2Cl2 + 2H2O → H2CrO4 + 2HCl
Its high reactivity toward water is further indicated by the fact that CrO2Cl2 fumes in moist air.
Chromyl chloride test for chloride
The chromyl chloride test entails heating a sample suspected of containing chloride with potassium dichromate and concentrated sulfuric acid. If chloride is present, chromyl chloride is formed and red fumes of CrO2Cl2 are evident. If there is no chloride present, no red fumes are produced. No analogous compounds are formed with fluorides, bromides, iodides and cyanides, so this test is therefore specific for chlorides. The test is related to the synthesis shown above, exposure of CrO42− to HCl.
Reagent for oxidation of alkenes
Depending on solvent, CrO2Cl2 oxidizes terminal alkenes to aldehydes. Internal alkenes give alpha-chloroketones or related derivatives.[4] It will also attack benzylic methyl groups to give aldehydes via the Étard reaction. Apart from this it can also be used for testing the absence of nitrate ions
Compatible solvents
CrO2Cl2 is such an aggressive reagent that solvents must be chosen judiciously. In light of its high reactivity toward water, CrO2Cl2 can be expected to decompose upon exposure to alcohols, similar to the behavior of other highly electrophilic chlorides such as VOCl3, TiCl4, and SO2Cl2. Typical for other electrophilic chlorides, chlorocarbons are excellent solvents, especially dichloromethane
As a further practical complication, chromyl chloride attacks most greases.
Safety considerations
CrO2Cl2 reacts with water to release hydrochloric acid (HCl) and hexavalent chromium (CrVI)
Acute: HCl can be acutely lethal. Exposure to chromyl chloride vapour irritates the respiratory system and severely irritates the eyes, and the liquid burns the skin and eyes. Ingestion would cause severe internal damage.[5]
Chronic: CrVI can produce chromosomal aberrations and is a human carcinogen via inhalation.[6] Frequent exposure of the skin to chromyl chloride may result in ulceration.[5]
Thus, CrO2Cl2 should be carefully handled in a well ventilated area. CrO2Cl2 is so aggressive that its storage can be problematic as it attacks rubber and most plastics as well as greases.
References
| Wikimedia Commons has media related to Chromyl chloride. |
- 1 2 3 4 5 6 7 8 "NIOSH Pocket Guide to Chemical Hazards #0142". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Moody, B.J. (1965). "22". Comparative Inorganic Chemistry (1 ed.). London: Edward Arnold. p. 381. ISBN 0-7131-3679-0.
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ↑ F. Freeman, R. H. DuBois, T. G. McLaughlin. "Aldehydes by Oxidation of Terminal Olefins with Chromyl Chloride: 2,4,4-Trimethylpentanal". Org. Synth.; Coll. Vol., 6, p. 1028
- 1 2 Prof CH Gray, ed. (1966). "IV". Laboratory Handbook of Toxic Agents (2 ed.). London: Royal Institute of Chemistry. p. 79.
- ↑ IARC (1999-11-05) [1990]. Volume 49: Chromium, Nickel, and Welding (PDF). pp. 21–23. ISBN 92-832-1249-5. Retrieved 2008-03-26.
Further reading
- F. Freeman "Chromyl Chloride" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289.