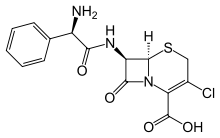
Cefaclor
 | |
| Clinical data | |
|---|---|
| Trade names | Biocef, Ceclor, Medacef,Distaclor, Keflor, Raniclor |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682729 |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | J01DC04 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well absorbed, independent of food intake |
| Metabolism | 15% to 40% |
| Biological half-life | 0.6 to 0.9 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
53994-73-3 |
| PubChem (CID) | 51038 |
| DrugBank |
DB00833 |
| ChemSpider |
46260 |
| UNII |
3Z6FS3IK0K |
| KEGG |
D00256 |
| ChEMBL |
CHEMBL8867 |
| ECHA InfoCard | 100.053.536 |
| Chemical and physical data | |
| Formula | C15H14ClN3O4S |
| Molar mass | 367.808 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Cefaclor, developed by Eli Lilly under the trade name Ceclor, is a second-generation cephalosporin antibiotic used to treat some infections caused by bacteria such as pneumonia and infections of the ear, lung, skin, throat, and urinary tract. It is also available from other manufacturers as a generic.[1]
Indications
Cefaclor belongs to the family of antibiotics known as the cephalosporins (cefalosporins). The cephalosporins are broad-spectrum antibiotics that are used for the treatment of septicaemia, pneumonia, meningitis, biliary tract infections, peritonitis, and urinary tract infections. The pharmacology of the cephalosporins is similar to that of the penicillins, excretion being principally renal. Cephalosporins penetrate the cerebrospinal fluid poorly unless the meninges are inflamed; cefotaxime is a more suitable cephalosporin than cefaclor for infections of the central nervous system, e.g. meningitis. Cefaclor is active against many bacteria, including both Gram-negative and Gram-positive organisms.
Cautions and contraindications
Cautions include known sensitivity to beta-lactam antibacterials, such as penicillins (Cefaclor should be avoided if there is a history of immediate hypersensitivity reaction); renal impairment (no dose adjustment required, although manufacturer advises caution); pregnancy and breast-feeding (but appropriate to use); false positive urinary glucose (if tested for reducing substances) and false positive Coombs test. Cefaclor has also been reported to cause a serum sickness-like reaction in children.[2][3]
Cefaclor is contraindicated in case of hypersensitivity (i.e. allergy) to cephalosporins.
Spectrum of bacterial susceptibility and resistance
Cefaclor is frequently used against bacteria responsible for causing skin infections, otitis media, urinary tract infections, and others. Cefaclor has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections: Gram positive aerobes - Staphylococci (including coagulase-positive, coagulase-negative, and penicillinase-producing strains), Streptococcus pneumoniae, and Streptococcus pyogenes (group A ß-hemolytic streptococci). [4] The following represents MIC susceptibility data for a few medically significant microorganisms.
- Haemophilus influenzae: 0.03 μg/mL - 128 μg/mL
- Staphylcoccus aureus: 0.6 μg/mL - 128 μg/mL
- Streptococcus pyogenes: 0.06 μg/mL - 4 μg/mL
Side effects
The principal side effect of the cephalosporins is hypersensitivity. Penicillin-sensitive patients will also be allergic to the cephalosporins, depending on the cephalosporin generation. The previous percentage of 10% cross reactivity rates are often overestimated. Allergic reactions may present as, for example, rashes, pruritus (itching), urticaria, serum sickness-like reactions with rashes, fever and arthralgia, and anaphylaxis. The frequency and severity of serum sickness-like reactions in children has led researchers to question its role in pediatric illness.[6]
Other side effects include gastrointestinal disturbances (e.g. diarrhea, nausea and vomiting, abdominal discomfort, disturbances in liver enzymes, transient hepatitis and cholestatic jaundice), headache, and Stevens–Johnson syndrome. Rare side effects include eosinophilia and blood disorders (including thrombocytopenia, leucopenia, agranulocytosis, aplastic anaemia and haemolytic anaemia); reversible interstitial nephritis; hyperactivity, nervousness, sleep disturbances, hallucinations, confusion, hypertonia, and dizziness. Toxic epidermal necrolysis has been reported. In the UK, The Committee on the Safety of Medicines (CSM) has warned that the risk of diarrhea and rarely antibiotic-associated colitis are more likely with higher doses.
Interactions with other medications
Coumarins
Cephalosporins possibly enhance the anticoagulant effect of coumarins (e.g. Warfarin) - change in patient's clinical condition, particularly associated with liver disease, intercurrent illness, or drug administration, necessitates more frequent testing of INR, and dose adjustment as necessary.
Probenecid
Excretion of cephalosporins is reduced by probenecid (resulting in increased concentrations of drug in the blood plasma).
Antacids
Absorption of cefaclor is reduced by H2 blockers (a type of antacid); therefore antacids should not be taken at the same time as cefaclor.
Safety in pregnancy and breastfeeding
Cefaclor is passed into the breast milk in small quantities, but is generally accepted to be safe to take during breastfeeding.[7] Cefaclor is not known to be harmful in pregnancy.[8]
Cefaclor CD
Cefaclor CD is a sustained release form of Cefaclor which releases the drug to the body over a longer period of time, which means that doses can be taken less frequently, with steadier levels of the drug in the bloodstream. Sustained release is useful with Cefaclor as it has a very short half-life. Blood transfusions are the #1 passer of this drug
References
- ↑ https://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm
- ↑ Hebert A, Sigman E, Levy M (1991). "Serum sickness-like reactions from cefaclor in children". J Am Acad Dermatol. 25 (5 Pt 1): 805–8. doi:10.1016/S0190-9622(08)80973-5. PMID 1802903.
- ↑ Parra F, Igea J, Martín J, Alonso M, Lezaun A, Sainz T (1992). "Serum sickness-like syndrome associated with cefaclor therapy". Allergy. 47 (4 Pt 2): 439–40. doi:10.1111/j.1398-9995.1992.tb02086.x. PMID 1456417.
- ↑ http://www.medicatione.com/?c=ing&s=cefaclor
- ↑ http://www.toku-e.com/Assets/MIC/Cefaclor.pdf
- ↑ King BA, Geelhoed GC (December 2003). "Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions". J Paediatr Child Health. 39 (9): 677–81. doi:10.1046/j.1440-1754.2003.00267.x. PMID 14629499.
- ↑ LactMED. "Summary of Cefaclor's use during lactation". National Library of Medicine. Retrieved 22 May 2011.
- ↑ Ito, S.; Blajchman A; Stephenson M; et al. (1993). "Prospective follow-up of adverse reactions in breast-fed infants exposed to maternal medication". Am J Obstet Gynecol. 168 (5): 1393–1399. doi:10.1016/s0002-9378(11)90771-6. PMID 8498418.