Canavanine
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Canavanine | |
| Systematic IUPAC name
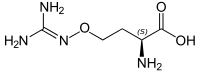
(2S)-2-amino-4-{[(diaminomethylidene)amino]oxy}butanoic acid | |
| Identifiers | |
| 543-38-4 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:609827 |
| ChemSpider | 388342 |
| DrugBank | DB01833 |
| ECHA InfoCard | 100.153.281 |
| KEGG | C00308 |
| MeSH | Canavanine |
| PubChem | 275 |
| |
| |
| Properties | |
| C5H12N4O3 | |
| Molar mass | 176.18 g·mol−1 |
| Density | 1.61 g·cm−3 (predicted) |
| Melting point | 184 °C (363 °F; 457 K) |
| Boiling point | 366 °C (691 °F; 639 K) |
| soluble | |
| Solubility | insoluble in alcohol, ether, benzene |
| log P | -0.91 (predicted) |
| Vapor pressure | 1.61 μPa (predicted) |
| Hazards | |
| Flash point | 214.6 °C (418.3 °F; 487.8 K) (predicted) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
L-(+)-(S)-Canavanine is a non-proteinogenic amino acid found in certain leguminous plants. It is structurally related to the proteinogenic α-amino acid L-arginine, the sole difference being the replacement of a methylene bridge (-CH
2- unit) in arginine with an oxa group (i.e., an oxygen atom) in canavanine. Canavanine is accumulated primarily in the seeds of the organisms which produce it, where it serves both as a highly deleterious defensive compound against herbivores and a vital source of nitrogen for the growing embryo[1] (see also L-canaline). The mechanism of canavanine's toxicity is that organisms that consume it typically mistakenly incorporate it into their own proteins in place of L-arginine, thereby producing structurally aberrant proteins that may not function properly.

Some specialized herbivores tolerate L-canavanine either because they metabolize it efficiently (cf. L-canaline) or avoid its incorporation into their own nascent proteins. An example of this ability can be found in the larvae of the tobacco budworm Heliothis virescens, which can tolerate massive amounts of dietary canavanine. These larvae fastidiously avoid incorporation of L-canavanine into their nascent proteins (presumably by virtue of highly discriminatory Arginine—tRNA ligase, the enzyme responsible for the first step in the incorporation of arginine into proteins). In contrast, larvae of the tobacco hornworm Manduca sexta can only tolerate tiny amounts (1.0 microgram per kilogram of fresh body weight[2]) of dietary canavanine because their arginine-tRNA ligase has little, if any, discriminatory capacity. No one has examined experimentally the arginine-tRNA synthetase of these organisms. But comparative studies of the incorporation of radiolabeled L-arginine and L-canavanine have shown that in Manduca sexta, the ratio of incorporation is about 3 to 1.
Dioclea megacarpa seeds contain high levels of canavanine. The beetle Caryedes brasiliensis is able to tolerate this however as it has the most highly discriminatory arginine-tRNA ligase known. In this insect, the level of radiolabeled L-canavanine incorporated into newly synthesized proteins is barely measurable. Moreover, this beetle uses canavanine as a nitrogen source to synthesize its other amino acids to allow it to develop.[3]
NZB/W F1, NZB, and DBA/2 mice fed L-canavanine develop a syndrome similar to systemic lupus erythematosus,[4] while BALB/c mice fed a steady diet of protein containing 1% canavanine showed no change in lifespan.[5] The toxicity of canavanine may be enhanced under conditions of protein starvation,[4] and canavanine toxicity resulting from consumption of Hedysarum alpinum seeds, which contain quantities of canavanine around 1%, has been implicated in the death of Christopher McCandless.[6]
Alfalfa seeds and sprouts contain L-canavanine. The L-canavanine in alfalfa has been linked to lupus-like symptoms in primates, including humans, and other auto-immune diseases. Often stopping consumption reverses the problem.[7][8][9]
See also
References
- ↑ "Non-protein amino acids (NPA)". January 2009. Archived from the original on 2011-01-22. Retrieved 2011-01-22.
- ↑ Rosenthal, G. A.; Dahlman, D. L. (1986). "L-Canavanine and protein synthesis in the tobacco hornworm Manduca sexta". Proceedings of the National Academy of Sciences. 83 (1): 14–8. Bibcode:1986PNAS...83...14R. doi:10.1073/pnas.83.1.14. JSTOR 26787. PMC 322781
 . PMID 3455753.
. PMID 3455753. - ↑ Rosenthal, G. A.; Hughes, C. G.; Janzen, D. H. (1982). "L-Canavanine, a Dietary Nitrogen Source for the Seed Predator Caryedes brasiliensis (Bruchidae)". Science. 217 (4557): 353–5. Bibcode:1982Sci...217..353R. doi:10.1126/science.217.4557.353. PMID 17791516.
- 1 2 Akaogi, Jun; Barker, Tolga; Kuroda, Yoshiki; Nacionales, Dina C.; Yamasaki, Yoshioki; Stevens, Bruce R.; Reeves, Westley H.; Satoh, Minoru (2006). "Role of non-protein amino acid l-canavanine in autoimmunity". Autoimmunity Reviews. 5 (6): 429–35. doi:10.1016/j.autrev.2005.12.004. PMID 16890899.
- ↑ Brown, Dan L (2005). "Canavanine-induced longevity in mice may require diets with greater than 15.7% protein". Nutrition & Metabolism. 2 (1): 7. doi:10.1186/1743-7075-2-7. PMC 554090
 . PMID 15733319.
. PMID 15733319. - ↑ Krakauer, Jon. "How Chris McCandless Died: An Update". The New Yorker. Retrieved 12 February 2015.
- ↑ Montanaro, A; Bardana Jr, E. J. (1991). "Dietary amino acid-induced systemic lupus erythematosus". Rheumatic diseases clinics of North America. 17 (2): 323–32. PMID 1862241.
- ↑ Herbert, V; Kasdan, T. S. (1994). "Alfalfa, vitamin E, and autoimmune disorders". The American Journal of Clinical Nutrition. 60 (4): 639–40. PMID 8092103.
- ↑ http://vegpeace.org/rawfoodtoxins.html[][]
Bibliography
- Rosenthal, Gerald A. (1986). "Biochemical insight into insecticidal properties ofl-Canavanine, a higher plant protective allelochemical". Journal of Chemical Ecology. 12 (5): 1145–56. doi:10.1007/BF01639001. PMID 24307052.
- Rosenthal, G. A.; Berge, M. A.; Bleiler, J. A.; Rudd, T. P. (1987). "Aberrant, canavanyl protein formation and the ability to tolerate or utilize L-canavanine". Experientia. 43 (5): 558–61. doi:10.1007/BF02143585. PMID 3582574.