Butein
 | |
| Names | |
|---|---|
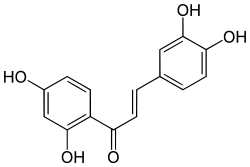
| IUPAC name
(E)-1-(2,4-dihydroxyphenyl)-3-(3,4-dihydroxyphenyl)prop-2-en-1-one | |
| Other names
2',3,4,4'-Tetrahydroxychalcone 2',4',3,4-Tetrahydroxychalcone 3,4,2',4'-Tetrahydroxychalcone 2′,4′,3,4-Tetrahydroxychalcone | |
| Identifiers | |
| 487-52-5 21849-70-7 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL128000 |
| ChemSpider | 4444634 |
| ECHA InfoCard | 100.006.963 |
| PubChem | 5281222 |
| |
| |
| Properties | |
| C15H12O5 | |
| Molar mass | 272.25 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Butein is a chalconoid. It can be found in Toxicodendron vernicifluum (or formerly Rhus verniciflua), Dahlia, Butea (Butea monosperma) and Coreopsis[1] It has antioxidative, aldose reductase and advanced glycation endproducts inhibitory effects.[2] It is also a sirtuin-activating compound, a chemical compound having an effect on sirtuins, a group of enzymes that use NAD+ to remove acetyl groups from proteins. It turned out that buteins possess a high ability to inhibit aromatase process in the human body, for this reason, the use of these compounds in the treatment of breast cancer on the estrogen ground has been taken into account.[3] The first attempts of sport pro-hormone supplementation with the use of buteins took place in Poland.[4]
References
- ↑ Semwal, R. B., Semwal, D. K., Combrinck, S., & Viljoen, A. (2015). Butein: From ancient traditional remedy to modern nutraceutical. Phytochemistry Letters, 11, 188-201. doi:10.1016/j.phytol.2014.12.014
- ↑ Lee EH, Song DG, Lee JY, Pan CH, Um BH, Jung SH (August 2008). "Inhibitory effect of the compounds isolated from Rhus verniciflua on aldose reductase and advanced glycation endproducts". Biol. Pharm. Bull. 31 (8): 1626–30. doi:10.1248/bpb.31.1626. PMID 18670102.
- ↑ Wang Y. "The plant polyphenol butein inhibits testosterone-induced proliferation in breast cancer cells expressing aromatase" Life Sci. 2005 May 20;77(1):39-51.
- ↑ S.Amboziak "Aromatase in the dock" 09.2012
This article is issued from Wikipedia - version of the 6/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.