Bullvalene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tricyclo[3.3.2.02,8]deca-3,6,9-triene | |||
| Other names
Bullvalen | |||
| Identifiers | |||
| 1005-51-2 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 120549 | ||
| PubChem | 136796 | ||
| UNII | ZQZ1X09E2B | ||
| |||
| |||
| Properties | |||
| C10H10 | |||
| Molar mass | 130.19 g/mol | ||
| Melting point | 96 °C (205 °F; 369 K) | ||
| Boiling point | decomposition at about 400 °C (752 °F; 673 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Bullvalene is a hydrocarbon with the chemical formula C10H10 with the unusual property that the chemical bonds making up the molecule are constantly rearranging as in fluxional molecules. For this reason bullvalene is extensively studied in organic chemistry.
Origin of the name
The name bullvalene is derived from the nickname of one the scientists who predicted its properties in 1963 and the underlying concept of valence tautomerism,[1] William "Bull" Doering.[2][3] According to Klärner in 2011, the weekly seminars organised by Doering were secretly called "Bull sessions" by PhD students and postdocs and "were feared by those who were poorly prepared".[4] The name was bestowed on the molecule, in 1961, by two of Doering's Yale graduate students, Maitland Jones and Ron Magid. The name celebrates Bill Doering's well-known nickname and was chosen to rhyme with fulvalene, a molecule of great interest to the research group.[5]
Stereodynamics
The bullvalene molecule is a cyclopropane platform with three vinyl arms conjoined at a methine group. As a fluxional molecule, bullvalene is subject to degenerate Cope rearrangements, with the result that all carbon atoms and hydrogen atoms appear equivalent on the NMR timescale. The number of possible valence tautomers is 10, since any of the 10 carbon atoms may be at the "apex".
 Scheme 1. Five of the bullvalene tautomers and some Cope rearrangements between them.
Scheme 1. Five of the bullvalene tautomers and some Cope rearrangements between them.
The compound was first synthesised in 1963 by G. Schröder by photolysis of a dimer of cyclooctatetraene with expulsion of benzene.
The unusual dynamic properties of bullvalene have been examined by dynamic NMR spectroscopy. For example, the proton NMR spectrum changes from a sharp singlet at 4.2 ppm at higher temperatures to a complex pattern at lower temperatures. This pattern is consistent with an exchange process whose rate k is close to the frequency separation of the four contributing resonances.
Related compounds
Bullvalones
In bullvalones one vinyl group in one of the arms in bullvalene is replaced by a keto group on a methylene bridge. In this way it is possible to activate the fluxional state by adding base and deactivate it again by removing the base:[6]
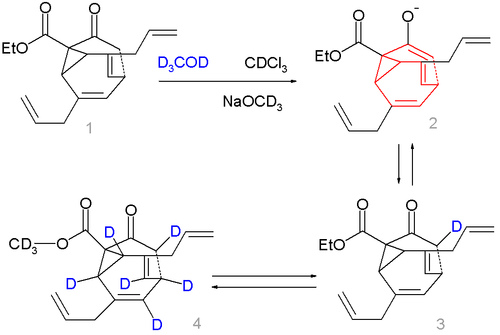
Compound 1 in scheme 2 is not a fluxional molecule but by adding base (sodium methoxide in methanol) the ketone converts to the enolate 2 and the fluxional state is switched on. Deuterium labeling is possible forming first 3 a then a complex mixture with up to 7 deuterium atoms, compound 4 being just one of them.
Semibullvalene
In semibullvalene (C8H8), one ethylene arm is replaced by a single bond. The compound was first prepared by photolysis of barrelene in isopentane with acetone as a photosensitizer in 1966.[7]

Semibullvalene exists only as two valence tautomers (2a and 2b in scheme 3) but in this molecule the Cope rearrangement takes place even at -110 °C, a temperature at which this type of reaction is ordinarily not possible.
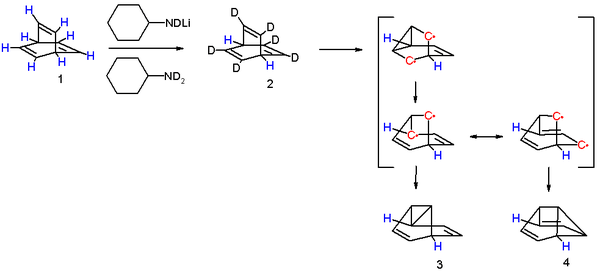
One insight into the reaction mechanism for this photoreaction is given by an isotope scrambling experiment.[8] The 6 vinylic protons in barrelene 1 are more acidic than the two bridgehead protons and therefore they can be replaced by deuterium with N-deuteriocyclohexylamide. Photolysis of 2 results in the initial formation of an biradical intermediate with a cyclopropane ring formed. This product rearranges to a second intermediate with a more favorable allylic radical as two mesomers. Intersystem crossing and radical recombination results in equal quantities of semibullvalenes 3 and 4. The new proton distribution with allylic, vinylic and cyclopropanyl protons determined with proton NMR confirms this model. As noted, the conversion of barrelene to semibullvalene is a di-pi-methane rearrangement.
A synthetic procedure for alkylated semibullvalenes published in 2006 is based on cyclodimerisation of a substituted 1,4-dilithio-1,3-butadiene with copper(I) bromide.[9] At 140 °C the ethylated semibullvalene isomerises to the cyclooctatetraene derivative.

Barbaralane
In barbaralane one ethylene arm is replaced by a methylene bridge and the dynamics are comparable to that of semibullvalene. There is also an intermediate ketone in bullvallene synthesis called "barbaralone". Both are named after Barbara M. Ferrier[10] (1932–2006) professor of the Department of Biochemistry and Biomedical Sciences at McMaster University.[11]
References
- ↑ Doering, W. von E.; Roth, W. R. (1963). "A Rapidly Reversible Degenerate Cope Rearrangement : Bicyclo[5.1.0]octa-2,5-diene". Tetrahedron. 19 (5): 715–737. doi:10.1016/S0040-4020(01)99207-5.
- ↑ Ault, Addison (2001). "The Bullvalene Story. The Conception of Bullvalene, a Molecule That Has No Permanent Structure". J. Chem. Educ. 78 (7): 924. doi:10.1021/ed078p924. Archived from the original on 2007-09-28.
- ↑ Author Ault (2001) also suggests the name stems from BS because of an unimpressed grad student
- ↑ Klärner, F.-G. (2011), William von Eggers Doering (1917–2011). Angewandte Chemie International Edition, 50: 2885–2886. doi: 10.1002/anie.201100453
- ↑ Nickon, A.; Silversmith, E. F. Organic Chemistry: The Name Game; Pergamon: New York, 1972; p 131.
- ↑ Lippert, A. R.; Kaeobamrung, J.; Bode, J. W. (2006). "Synthesis of Oligosubstituted Bullvalones: Shapeshifting Molecules Under Basic Conditions". J. Am. Chem. Soc. 128 (46): 14738–14739. doi:10.1021/ja063900+. PMID 17105247.
- ↑ Zimmerman, H. E.; Grunewald, G. L. (1966). "The Chemistry of Barrelene. III. A Unique Photoisomerization to Semibullvalene". J. Am. Chem. Soc. 88 (1): 183–184. doi:10.1021/ja00953a045.
- ↑ Zimmerman, H. E.; Binkley, R. W.; Givens, R. S.; Sherwin, M. A. (1967). "Mechanistic Organic Photochemistry. XXIV. The Mechanism of the Conversion of Barrelene to Semibullvalene. A General Photochemical Process" (PDF). J. Am. Chem. Soc. 89 (15): 3932–3933. doi:10.1021/ja00991a064.
- ↑ Wang, C.; Yuan, J.; Li, G.; Wang, Z.; Zhang, S.; Xi, Z. (2006). "Metal-Mediated Efficient Synthesis, Structural Characterization, and Skeletal Rearrangement of Octasubstituted Semibullvalenes". J. Am. Chem. Soc. 128 (14): 4564–4565. doi:10.1021/ja0579208. PMID 16594680.
- ↑ Alex Nickon, Ernest F. Silversmith, Organic Chemistry: The Name Game: Modern Coined Terms and Their Origins, p. 133, Pergamon Press, 1987.
- ↑ A tribute to professor emeritus Barbara Ferrier, McMaster University, 6 January 2006

