Bridged compounds

_V1.svg.png)
A bridged compound is a chemical compound, generally an organic compound, but also possibly inorganic, that has two or more rings (a ring system) that contains a bridge—a single atom or an unbranched chain of atoms (or even just a valence bond) that connect two "bridgehead" atoms. The bridgehead atoms are defined as any atom that is not a hydrogen, and that is part of the skeletal framework of the molecule that is bonded to three or more other skeletal atoms.[1]:VB-1 Clayden simplifies this description, saying that bridged bicyclic compounds "are just what the name implies—[molecules in which] a bridge of [an atom or of atoms] is thrown across from one side of the ring to the other,"[2]:839 examples of which are shown at left and right, and below. The presence of the bridge connecting the bridgehead atoms, which are most often two non-adjacent atoms, distinguishes bridged compounds from fused ring compounds that have two rings linked by two adjacent atoms, and from spiro compounds that have two rings linked by a single atom.[2]:653ff :839ff
Bridged compounds may be fully carbocyclic (all carbon) or heterocyclic (having one or more non-carbon atoms). The principle and bridging rings are almost always different in nature, though they can be identical. Although sketches of organic structures may make bridged compounds appear planar, they are not; for instance, comparing the 3D ball-and-stick model of norbornane at left, with the image shown at right, makes clear the bridging ring ensures that the molecule is not planar.
Nomenclature

The nomenclature of bridged compounds was established by the IUPAC based on the system introduced by von Baeyer.[3] Some important concepts and definitions for bridged compounds are as follows.
- bridgehead refers to any skeletal atom of the ring system that participates in chemical bonds to 3 or more other non-hydrogen skeletal atoms. Hence, in fully carbocyclic bridged compounds, bridgehead atoms are always tertiary or quaternary carbon atoms;[1]:VB-1
- a bridge can refer either to a single valence bond that connects two bridgehead atom, or to a chain of atoms between bridgeheads that is unbranched, where the atoms can be carbon atoms or heteroatom (but because at least divalent, not hydrogen);[1]:VB-1
- the main ring refers to a ring that is a part of the ring system, and that is chosen to "include as many skeletal atoms of the polycyclic compound as possible"; i.e., it is most often the ring with the largest number of continuous atoms (carbon or heteroatoms).[1]:VB-2 For instance, in the case of norbornane, shown above, while both cyclohexane and cyclopentane rings are evident within it, the main ring is the cyclohexane ring.
- the main bridge refers to a bridge that connects two bridgeheads of the main ring, where the main bridge of norbornane is its only bridge, the one atom connecting the two bridgehead atoms (indicated at right, above, by the arrows);[1]:VB-1 and
- secondary bridge refers to any other bridges in the ring system that are not the main bridge of the main ring.[1]:VB-1
Examples
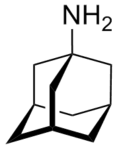
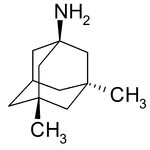
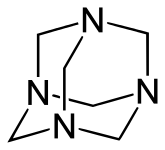
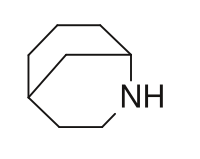
Examples include: norbornane, shown above, along with other alicyclic (purely hydrocarbon) examples in the Nomenclature section; adamantane and its amine analogs memantine (shown), amantadine (shown), and rimantadine (the latter two historic influenza drugs); the heteroatom bridgehead examples hexamine (shown) and 1,4- Diazabicyclo[2.2.2]octane (DABCO, shown in two representations); morphan, an example having a heteroatom in a bridge (shown); biperiden; and methenamine.
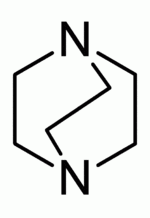 DABCO, alternative representation of bicyclic rings |
Further reading
The following are texts and other sources covering the title subject.
Secondary sources
- Well-regarded intermediate textbook of organic chemistry: Clayden, Jonathan ; Greeves, Nick & Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford, UK: Oxford University Press. pp. 432ff, 653ff, 839ff, and passim. ISBN 0199270295. Retrieved 2 February 2016.
- Examples of inorganic bridged compounds. Sita, Lawrence R. & Kinoshita, Isamu (2012). "The Molecular and Electronic Structure of Polycyclic Polystannanes". In Harrod, J.F. & Laine, R.M. Inorganic and Organometallic Oligomers and Polymers: Proceedings of the 33rd IUPAC Symposium on Macromolecules. Berlin, GER: Springer Science & Business. pp. 115–126. ISBN 9401132143. Retrieved 2 February 2016.
Primary sources
- Examples of inorganic bridged compounds. S. K. Xi, S.K.; Schmidt, H.; Lensink, C.; Kim, S.; Wintergrass, D.; Daniels, L. M.; Jacobson, R. A. & Verkade, John G. (1990). "Bridgehead-bridgehead communication in untransannulated phosphatrane ZP(ECH2CH2)3N systems" (print, online research report). Inorg. Chem. 29 (12; June): 2214–2220. doi:10.1021/ic00337a008. Retrieved 1 March 2016.
Notes and references
- 1 2 3 4 5 6 Moss, G.P. and the Working Party of the International Union of Pure and Applied Chemistry [IUPAC], Organic Chemistry Division, Commission on Nomenclature of Organic Chemistry (III.1) (1999). "Extension and Revision of the von Baeyer System for Naming Polycyclic Compounds (Including Bicyclic Compounds); (IUPAC Recommendations 1999)" (PDF). Pure Appl. Chem. 71 (3): 513–529. doi:10.1351/pac199971030513. ISSN 1365-3075. Retrieved 3 February 2016. Note, the article co-authors, the Working Party of the IUPAC (1992-1998), were P. M. Giles, Jr., E. W. Godly, K.-H. Hellwich, A. K. Ikizler, M. V. Kisakürek, A. D. McNaught, G. P. Moss, J. Nyitrai, W. H. Powell, O. Weissbach, and A. Yerin. Also available online at "Extension and Revision… [as above]". London, GBR: Queen Mary University of London. Retrieved 3 February 2016. Also available in German, with et al. indicating the same working party, at Hellwich, Karl-Heinz et al. (2002). "Erweiterung und Revision des von-Baeyer-Systems zur Benennung polycyclischer Verbindungen (einschließlich bicyclischer Verbindungen)". Angewandte Chemie. 114 (17, September 2): 3423–3432. doi:10.1002/1521-3757(20020902)114:17<3423::AID-ANGE3423>3.0.CO;2-6. Retrieved 3 February 2016.
Die Übersetzung basiert auf der „Extension and Revision of the von Baeyer System for Naming Polycyclic Compounds (Including Bicyclic Compounds)“ der Commission on Nomenclature of Organic Chemistry (III.1) der Organic Chemistry Division der International Union of Pure and Applied Chemistry, veröffentlicht in Pure Appl. Chem.1999, 71, 513–529.
- 1 2 Clayden, Jonathan ; Greeves, Nick & Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford, UK: Oxford University Press. pp. 432ff, 653ff, 839ff, and passim. ISBN 0199270295. Retrieved 2 February 2016.
- ↑ "Von-Baeyer-System". chemie.de. Retrieved 2 March 2016.
See also
External links
- Bridged Compounds at the US National Library of Medicine Medical Subject Headings (MeSH)
- IUPAC Blue Book Rule A-31. Bridged hydrocarbons. Bicyclic Systems
- IUPAC Blue Book Rule A-32. Bridged Hydrocarbons. Polycyclic Systems
- IUPAC Blue Book Rule A-34. Hydrocarbon Bridges. Bridged Hydrocarbons
- IUPAC Blue Book Rule B-14. Bridged Heterocyclic Systems. Extension of the von Baeyer System.