Biginelli reaction
| Biginelli reaction | |
|---|---|
| Named after | Pietro Biginelli |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | biginelli-reaction |
| RSC ontology ID | RXNO:0000236 |
The Biginelli reaction is a multiple-component chemical reaction that creates 3,4-dihydropyrimidin-2(1H)-ones 4 from ethyl acetoacetate 1, an aryl aldehyde (such as benzaldehyde 2), and urea 3.[1][2][3][4] It is named for the Italian chemist Pietro Biginelli.[5][6]
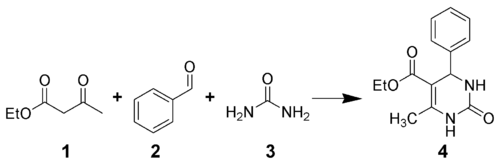
This reaction was developed by Pietro Biginelli in 1891. The reaction can be catalyzed by Brønsted acids and/or by Lewis acids such as copper(II) trifluoroacetate hydrate[7] and boron trifluoride.[8] Several solid-phase protocols utilizing different linker combinations have been published.[9][10]
Dihydropyrimidinones, the products of the Biginelli reaction, are widely used in the pharmaceutical industry as calcium channel blockers,[11] antihypertensive agents, and alpha-1-a-antagonists.
Reaction mechanism
The reaction mechanism of the Biginelli reaction is a series of bimolecular reactions leading to the desired dihydropyrimidinone.[12]
According to a mechanism proposed by Sweet in 1973 the aldol condensation of ethylacetoacetate 1 and the aryl aldehyde is the rate-limiting step leading to the carbenium ion 2. The nucleophilic addition of urea gives the intermediate 4, which quickly dehydrates to give the desired product 5.[13]

This mechanism is superseded by one by Kappe in 1997:
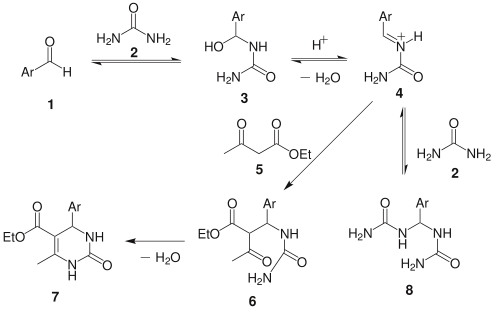
This scheme begins with rate determining nucleophilic addition by the urea to the aldehyde.[14][15] The ensuing condensation step is catalyzed by the addition of acid, resulting in the imine nitrogen. The β-ketoester then adds to the imine bond and consequently the ring is closed by the nucleophilic attack by the amine onto the carbonyl group. This final step ensues a second condensation and results in the Biginelli compound.
Advances in Biginelli reaction
In 1987, Atwal et al.[16][17] reported a modification to the Biginelli reaction that consistently generated higher yields. Atul Kumar has reported first enzymatic synthesis for Biginelli reaction via yeast catalysed protocol in high yields.[18]
References
- ↑ Biginelli, P. (1891). "Ueber Aldehyduramide des Acetessigäthers". Chemische Berichte. 24: 1317. doi:10.1002/cber.189102401228.
- ↑ Biginelli, P. (1891). "Ueber Aldehyduramide des Acetessigäthers. II". Chemische Berichte. 24 (2): 2962. doi:10.1002/cber.189102402126.
- ↑ Zaugg, H. E.; Martin, W. B. (1965). "Α-Amidoalkylations at Carbon". Org. React. 14: 88. doi:10.1002/0471264180.or014.02. ISBN 0471264180.
- ↑ Kappe, C. O. (1993). "100 years of the biginelli dihydropyrimidine synthesis". Tetrahedron. 49 (32): 6937–6963. doi:10.1016/S0040-4020(01)87971-0.
- ↑ Kappe, C. Oliver (2005) "The Biginelli Reaction", in: J. Zhu and H. Bienaymé (eds.): Multicomponent Reactions, Wiley-VCH, Weinheim, ISBN 978-3-527-30806-4.
- ↑ Kappe, C. O.; Stadler, A. (2004). "The Biginelli Dihydropyrimidine Synthesis". Organic Reactions. 63. doi:10.1002/0471264180.or063.01. ISBN 0471264180.
- ↑ Song, Dailei; Wang, Runxia; Chen, Yongli; Zhang, Shaohua; Liu, Chunsheng; Luo, Genxiang (2008). "Copper(II) trifluoroacetate catalyzed synthesis of 3,4- dihydropyrimidin-2(1H)-ones under solvent-free conditions". Reaction Kinetics and Catalysis Letters. 95 (2): 385. doi:10.1007/s11144-008-5379-2.
- ↑ Hu, E. H.; Sidler, D. R.; Dolling, U.-H. (1998). "Unprecedented Catalytic Three Component One-Pot Condensation Reaction: An Efficient Synthesis of 5-Alkoxycarbonyl- 4-aryl-3,4-dihydropyrimidin-2(1H)-ones". J. Org. Chem. 63 (10): 3454. doi:10.1021/jo970846u.
- ↑ Wipf, P.; Cunningham, A. (1995). "A solid phase protocol of the biginelli dihydropyrimidine synthesis suitable for combinatorial chemistry". Tetrahedron Lett. 36 (43): 7819–7822. doi:10.1016/0040-4039(95)01660-A.
- ↑ Kappe, C. O. (2000). "Highly versatile solid phase synthesis of biofunctional 4-aryl-3,4-dihydropyrimidines using resin-bound isothiourea building blocks and multidirectional resin cleavage". Bioorg. Med. Chem. Lett. 10 (1): 49–51. doi:10.1016/S0960-894X(99)00572-7. PMID 10636241.
- ↑ Rovnyak, G. C.; Atwal, K. S.; Hedberg, A.; Kimball, S. D.; Moreland, S.; Gougoutas, J. Z.; O'Reilly, B. C.; Schwartz, J.; Malley, M. F. (1992). "Dihydropyrimidine calcium channel blockers. 4. Basic 3-substituted-4-aryl-1,4-dihydropyrimidine-5-carboxylic acid esters. Potent antihypertensive agents". J. Med. Chem. 35 (17): 3254–3263. doi:10.1021/jm00095a023.
- ↑ Folkers, K.; Johnson, T. B. (1933). "Researches on Pyrimidines. CXXXVI. The Mechanism of Formation of Tetrahydropyrimidines by the Biginelli Reaction1". J. Am. Chem. Soc. 55 (9): 3784–3791. doi:10.1021/ja01336a054.
- ↑ Sweet, F.; Fissekis, J. D. (1973). "Synthesis of 3,4-dihydro-2(1H)-pyrimidinones and the mechanism of the Biginelli reaction". J. Am. Chem. Soc. 95 (26): 8741–8749. doi:10.1021/ja00807a040.
- ↑ Folkers, K.; Harwood, H. J.; Johnson, T. B. (1932). "Researches on Pyrimidines. Cxxx. Synthesis of 2-Keto-1,2,3,4-Tetrahydropyrimidines". J. Am. Chem. Soc. 54 (9): 3751–3758. doi:10.1021/ja01348a040.
- ↑ Kappe, C.O. (1997). "A Reexamination of the Mechanism of the Biginelli Dihydropyrimidine Synthesis. Support for anN-Acyliminium Ion Intermediate1". J. Org. Chem. 62 (21): 7201–7204. doi:10.1021/jo971010u.
- ↑ O'Reilly, B. C.; Atwal, K. S. (1987). "Synthesis of Substituted 1,2,3,4-Tetrahydro-6-methyl-2-oxo-5-pyrimidinecarboxylic Acid Esters: The Biginelli Condensation Revisited". Heterocycles. 26 (5): 1185–1188. doi:10.3987/R-1987-05-1185.
- ↑ O'Reilly, B. C.; Atwal, K. S. (1987). "Synthesis of Substituted 1,2,3,4-Tetrahydro-6-methyl-2-thioxo-5-pyrimidinecarboxylic Acid Esters". Heterocycles. 26 (5): 1189–1192. doi:10.3987/R-1987-05-1189.
- ↑ Kumar, Atul; Maurya, Ram Awatar (2007). "An efficient bakers' yeast catalyzed synthesis of 3,4-dihydropyrimidin-2-(1H)-ones". Tetrahedron Letters. 48 (26): 4569. doi:10.1016/j.tetlet.2007.04.130.