Benzotriazole
 | |
 | |
| Names | |
|---|---|
| Other names
1H-Benzotriazole, 1,2,3-Benzotriazole, BtaH | |
| Identifiers | |
| 95-14-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:75331 |
| ChEMBL | ChEMBL84963 |
| ChemSpider | 6950 |
| ECHA InfoCard | 100.002.177 |
| PubChem | 7220 |
| |
| |
| Properties | |
| C6H5N3 | |
| Molar mass | 119.13 g·mol−1 |
| Appearance | White solid |
| Density | 1.36 g/mL [1] |
| Melting point | 100 °C (212 °F; 373 K)[2] |
| Boiling point | 350 °C (662 °F; 623 K)[2] |
| 20 g/L[2] | |
| Acidity (pKa) | 8.2 [3][4] |
| Basicity (pKb) | < 0 [4] |
| Hazards | |
| Main hazards | XN [1] |
| R-phrases | R20/22 R36 R52/53 [1] |
| S-phrases | – |
| R/S statement | – |
| Related compounds | |
| Related compounds |
Benzimidazole |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Benzotriazole (BTA) is a heterocyclic compound containing three nitrogen atoms, with the chemical formula C6H5N3. This aromatic compound is colorless and polar and can be used in various fields.
Structure
Benzotriazole features two fused rings. Its five-membered ring can exist in tautomers A and B, and the derivatives of both tautomers, structures C and D also can be produced.[5]
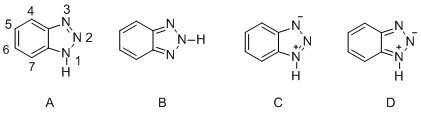
Various structural analyses with UV, IR and 1H-NMR spectra indicated that isomer A is predominantly present at room temperature. The bond between positions 1 and 2 and the one between positions 2 and 3 have proved to have the same bond properties. Moreover, the proton does not tightly bind to any of the nitrogen atoms, but rather migrates rapidly between positions 1 and 3. Therefore, the BTA can lose a proton to act as a weak acid (pKa = 8.2)[3][4] or accept a proton using the lone pair electrons located on its nitrogen atoms as a very weak Bronsted base (pKa < 0).[4] Not only can it act either as an acid or base, it can also bind to other species, utilizing the lone pair electrons. Applying this property, the BTA can form a stable coordination compound on a copper surface and behave as a corrosion inhibitor.[5]
Synthesis
A synthesis of the BTA involves the reaction of o-phenylenediamine, sodium nitrite and acetic acid. The conversion proceeds via diazotization of one of the amine groups.[6]
The synthesis can be improved when the reaction is carried out at low temperatures (5-10 ˚C) and briefly irradiated in an ultrasonic bath.[7]
Applications
Benzotriazole has been known for its great versatility. It has already been used as a restrainer in photographic emulsions and as a reagent for the analytical determination of silver. More importantly, it has been extensively used as a corrosion inhibitor in the atmosphere and underwater. Also, its derivatives and their effectiveness as drug precursors have been drawing attention. Besides all the application mentioned above, the BTA can be used as antifreezes, heating and cooling systems, hydraulic fluids and vapor phase inhibitors as well.[5]
Corrosion inhibitor
Benzotriazole is an effective corrosion inhibitor for copper and its alloys by preventing undesirable surface reactions. It is known that a passive layer, consisting of a complex between copper and benzotriazole, is formed when copper is immersed in a solution containing benzotriazole. The passive layer is insoluble in aqueous and many organic solutions. There is a positive correlation between the thickness of the passive layer and the efficiency of preventing corrosion.[8] BTA is used in conservation, notably for the treatment of bronze disease. The exact structure of the copper-BTA complex is controversial and many proposals have been suggested.
Drug precursor
Benzotriazole derivatives have chemical and biological properties that are versatile in the pharmaceutical industry. Benzotriazole derivatives act as agonists for many proteins. For instance, vorozole and alizapride have useful inhibitory properties against different proteins and benzotriazole esters have been reported to work as mechanism-based inactivators for severe acute respiratory syndrome (SARS) 3CL protease. The methodology is not only limited to heterocyclization but was also successful for polynuclear hydrocarbons of small carbocyclic systems.[9]
It is also used in photographic developers and emulsion as a restrainer.
Environmental relevance
Benzotriazole is fairly water-soluble, not readily degradable and has a limited sorption tendency. Hence, it is only partly removed in wastewater treatment plants and a substantial fraction reaches surface water such as rivers and lakes.[10]
References
- 1 2 3 chemdat.info
- 1 2 3 1H-Benzotriazole Archived September 27, 2007, at the Wayback Machine., SRC PhysProp Database
- 1 2 Katritzky, A. R.; Rachwal S.; Hitchings G. J. (14 January 1991). "Benzotriazole: A novel synthetic auxiliary". Tetrahedron. 47 (16–17): 2683–2732. doi:10.1016/S0040-4020(01)87080-0.
- 1 2 3 4 Katritzky, A. R. "Adventures with Benzotriazole" (PDF). Lecture presented at various locations in 2002. Florida Center for Heterocyclic CompoundsFor. Retrieved 23 November 2011.
- 1 2 3 Sease, Catherine (May 1978). "Benzotriazole: A Review for Conservators". Studies in Conservation. 2. 23 (2): 76–85. doi:10.2307/1505798. JSTOR 1505798.
- ↑ Robert A. Smiley "Phenylene- and Toluenediamines" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_405
- ↑ Claudio, M. P.; Pereira, Helio A.; Stefani, Karla P.; Guzen, Aline T. G. (29 August 2006). "Improved Synthesis of Benzotriazoles and 1-Acylbenzotriazoles by Ultrasound Irradiation" (PDF). Letters in Organic Chemistry. 4 (31): 43–46. doi:10.1002/chin.200731104. Retrieved 23 November 2011.
- ↑ Finšgar, M.; Milošev I. (11 March 2010). "Inhibition of copper corrosion by 1,2,3-benzotriazole: A review". Corrosion Science. 52 (9): 2737–2749. doi:10.1016/j.corsci.2010.05.002.
- ↑ Kale, Raju R.; Virendra Prasad; Prabhu P. Mohapatra; Vinod K. Tiwari (6 March 2010). "Recent developments in benzotriazole methodology for construction of pharmacologically important heterocyclic skeletons". Monatsh Chemistry. 141 (11): 1159–1182. doi:10.1007/s00706-010-0378-1.
- ↑ Giger, W; Schaffner, C; Kohler, HP (2006). "Benzotriazole and tolyltriazole as aquatic contaminants. 1. Input and occurrence in rivers and lakes". Environmental Science & Technology. 40 (23): 7186–92. doi:10.1021/es061565j. PMID 17180965.
| Wikimedia Commons has media related to Benzotriazole. |
