BamHI
| BamH I | |||||||||
|---|---|---|---|---|---|---|---|---|---|
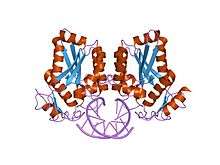 Restriction endonuclease BamH I bound to a non-specific DNA. | |||||||||
| Identifiers | |||||||||
| Symbol | BamH I | ||||||||
| Pfam | PF02923 | ||||||||
| Pfam clan | CL0236 | ||||||||
| InterPro | IPR004194 | ||||||||
| SCOP | 1bhm | ||||||||
| SUPERFAMILY | 1bhm | ||||||||
| |||||||||
BamH I (from Bacillus amyloliquefaciens) is a type II restriction endonuclease, having the capacity for recognizing short sequences (6 b.p.) of DNA and specifically cleaving them at a target site. This exhibit focuses on the structure-function relations of BamH I as described by Newman, et al. (1995). BamH I binds at the recognition sequence 5'-GGATCC-3', and cleaves these sequences just after the 5'-guanine on each strand. This cleavage results in sticky ends which are 4 b.p. long. In its unbound form, BamH I displays a central b sheet, which resides in between a helices. BamH I is an extraordinarily unique molecule in that it undergoes a series of unconventional conformational changes upon DNA recognition. This allows the DNA to maintain its normal B-DNA conformation without distorting to facilitate enzyme binding. BamH I is a symmetric dimer. DNA is bound in a large cleft that is formed between dimers; the enzyme binds in a "crossover" manner. Each BamH I subunit makes the majority of its backbone contacts with the phosphates of a DNA half site but base pair contacts are made between each BamH I subunit and nitrogenous bases in the major groove of the opposite DNA half site. The protein binds the bases through either direct hydrogen bonds or water-mediated H-bonds between the protein and every H-bond donor/acceptor group in the major groove. Major groove contacts are formed by atoms residing on the amino-terminus of a parallel 4 helix bundle. This bundle marks the BamH I dimer interface, and it is thought that the dipole moments of the NH2-terminal atoms on this bundle may contribute to electrostatic stabilization.
Sites of Recognition Between BamH I and DNA
The BamH I enzyme is capable of making a large number of contacts with DNA. Water-mediated hydrogen bonding, as well as both main-chain and side-chain interactions aid in binding of the BamH I recognition sequence. In the major groove, the majority of enzyme/DNA contacts take place at the amino terminus of the parallel-4-helix bundle, made up of a4 and a6 from each subunit. Although a6 from each subunit does not enter the DNA major groove, its preceding loops interact with the outer ends of the recognition site. Conversely, a4 from each subunit does enter the major groove in the center of the recognition sequence. A total of 18 bonds are formed between the enzyme and DNA across the 6 base pair recognition sequence (12 direct and 6 water mediated bonds). As discussed above, the L and R subunits bind in a cross over manner, whereby the R-subunit of BamH I contacts the left DNA half-site of the recognition sequence. The binding of each BamH I subunit is precisely the same as its symmetrical partner. The recognition site for BamH I has a palindromic sequence which can be cut in half for ease in showing bonds.
Recognition site
G G A T C C C C T A G G
As of the end of 2010, there were 5 crystal structures of BamH I in the Protein Data Bank
Two-metal Mechanism
BamHI, type II restriction endonucleases, often requires divalent metals as cofactors to catalyze DNA cleavage.[1] Two-metal ion mechanism is one of the possible catalytic mechanisms of BamHI since the BamHI crystal structure has the ability to bind two metal ions at the active site, which is suitable for the classical two-metal ion mechanism to proceed. Two-metal ion mechanism is the use of two metal ions to catalyze the cleavage reaction of restriction enzyme. BamHI has three critical active site residues that are important for metal catalyst. They are known as Asp94, Glu111 and Glu113. These residues are usually acidic. In the presence of a metal ion, the residues are pointed toward the metal ion. In the absence of metal ions, the residues are pointed outward. The two metal ions (A and B) are 4.1 apart from each other in the active site and are in-line with these residues.[2] In general, when the two metal ions (A and B) are bonded to the active site, they help stabilize a cluster distribution of negative charges localized at the active site created by the leaving of an oxygen atom during the transition state. First, a water molecule will be activated by metal ion A at the active site. This water molecule will act as the attacking molecule attacking the BamHI-DNA complex and thus making the complex negative. Later, another water will bound to metal ion B and donate a proton to the leaving group of complex, stabilizing the build-up of negative charge on the leaving oxygen atom.[3]
The function of Ca2+ in the active site of BamHI is known. It is an inhibitor of DNA cleavage, converting BamHI into the pre-reactive state. This revealed the water molecular is the attacking molecule. It donates a proton to the leaving group that is bounded to Ca2+ forming a 90o O-P-O bond angles. If Glu 113 is replaced by lysine, the cleavage is lost since Glu 113 accepts the proton from the attacking water molecule.[2]
References
- ↑ Ninfa, Alexander (2009). Fundamental Laboratory Approaches for Biochemistry and Biotechnology. Wiley. p. 345. ISBN 978-0470087664.
- 1 2 Viadiu, hector (1998). "The role of metals in catalysis by the restriction endonuclease BamHI". Nature Structural Biology.
- ↑ Mordasini, Tiziana (December 18, 2002). "Why do divalent metal ions either promote or inhibit enzymatic reactions? - The case of BamHI restriction endonuclease from combined quantum-classical simulations". The Journal of Biological Chemistry.
- M. Newman, T. Strzelecka, F.D. Dorner, I. Schildkraut, A. K. Aggarwal, Science. 269, 656 (1995)
External links
- Deoxyribonuclease BamHI at the US National Library of Medicine Medical Subject Headings (MeSH)
- 5 crystal structures
This article incorporates text from the public domain Pfam and InterPro IPR004194