Baker–Venkataraman rearrangement
The Baker–Venkataraman rearrangement is the chemical reaction of 2-acetoxyacetophenones with base to form 1,3-diketones.[1][2]

This rearrangement reaction proceeds via enolate formation followed by acyl transfer. It is named after the scientists Wilson Baker and Krishnasami Venkataraman.
The Baker–Venkataraman rearrangement is often used to synthesize chromones and flavones.[3][4][5][6][7][8][9][10]
Mechanism
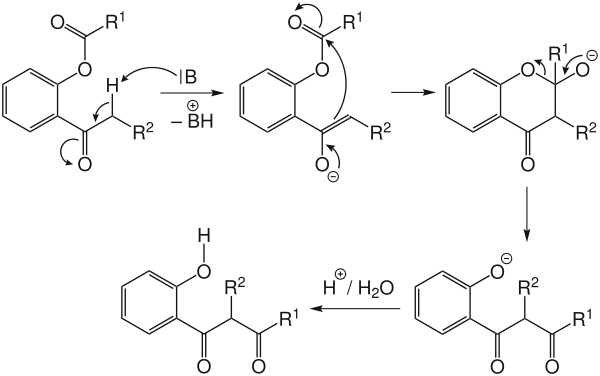
A base abstracts the hydrogen atom alpha to the aromatic ketone, forming an enolate. Then, the enolate attacks the ester carbonyl to form a cyclic alkoxide. The cyclic intermediate is opened up to form a more stable phenolate, which is protonated during acidic work-up to give the desired product.
See also
References
- ↑ Baker, W. (1933). "Molecular rearrangement of some o-acyloxyacetophenones and the mechanism of the production of 3-acylchromones". J. Chem. Soc.: 1381–1389. doi:10.1039/JR9330001381.
- ↑ Mahal, H. S.; Venkataraman, K. (1934). "Synthetical experiments in the chromone. group. XIV. Action of sodamide on 1-acyloxy-2-acetonaphthones". J. Chem. Soc.: 1767–1769. doi:10.1039/JR9340001767.
- ↑ Wheeler, T. S. (1952). "Flavone". Organic Syntheses 32: 72. (also in the Collective Volume (1963) 4: 478 (PDF)).
- ↑ Jain, P. K.; Makrandi, J. K.; et al. (1982). "A Facile Baker-Venkataraman Synthesis of Flavones using Phase Transfer Catalysis". Synthesis 1982 (3): 221–222. doi:10.1055/s-1982-29755.
- ↑ Kalinin, A. V.; Da Silva, A. J. M.; Lopes, C. C.; Lopes, R. S. C.; Snieckus, V. (1998). "Directed ortho metalation – cross coupling links. Carbamoyl rendition of the Baker-Venkataraman rearrangement. Regiospecific route to substituted 4-hydroxycoumarins". Tetrahedron Letters 39 (28): 4995–4998. doi:10.1016/S0040-4039(98)00977-0.
- ↑ Kraus, G. A.; Fulton, B. S. Wood, S.H. (1984). "Aliphatic acyl transfer in the Baker-Venkataraman reaction". J. Org. Chem. 49 (17): 3212. doi:10.1021/jo00191a033.
- ↑ Reddy, B.P.; Krupadanam, G.L.D. (1996). "The synthesis of 8-allyl-2-styrylchromones by the modified baker-venkataraman transformation". J. Heterocycl. Chem. 33 (6): 1561. doi:10.1002/jhet.5570330602.
- ↑ Kalinin, A.V.; Sneckus, V. (1998). "4,6-Dimethoxy-3,7-dimethylcoumarin from Colchicum decaisnei. Total synthesis by carbamoyl Baker-Venkataraman rearrangement and structural revision to isoeugenetin methyl ether". Tetrahedron Lett. 39 (28): 4999. doi:10.1016/S0040-4039(98)00978-2.
- ↑ Thasana, N.; Ruchirawat, S. (2002). "The application of the Baker–Venkataraman rearrangement to the synthesis of benz[b]indeno[2,1-e]pyran-10,11-dione". Tetrahedron Lett. 43 (25): 4515. doi:10.1016/S0040-4039(02)00818-3.
- ↑ Santos, C.M.M.; Silva, A.M.S. and Cavaleiro, J.A.S. (2003). "Synthesis of New Hydroxy-2-styrylchromones". Eur. J. Org. Chem. 2003 (23): 4575. doi:10.1002/ejoc.200300468.
External links
This article is issued from Wikipedia - version of the 10/24/2014. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.