Atmospheric-pressure chemical ionization
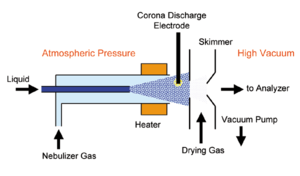
Atmospheric pressure chemical ionization (APCI) is an ionization method used in mass spectrometry (commonly LC-MS) which utilizes gas-phase ion-molecule reactions at atmospheric pressure.[1] It is an ionization method that is similar to chemical ionization (commonly used in GC-MS) where corona discharges on a solvent spray produce primary ions.[2] APCI is mainly used with polar and relatively nonpolar compounds with a molecular weight of less than 1500 Da, generally giving monocharged ions. Because of its ability to run in sequence with HPLC, it has gained a large popularity in trace analysis detection and pharmacology.[3]
Ionization
The analyte in solution is directly introduced into a pneumatic nebulizer via a direct inlet probe or through a connection to a standard high-performance liquid chromatography (HPLC) with an eluent flow rate between 0.2 and 2 mL/min. Once in the nebulizer, the analyte in solution is converted into a thin fog through the use of a high-speed nitrogen beam, and the droplets are then displaced by the gas flow through a heated quartz tube called a desolvation/vaporization chamber. [4]
The mobile phase and the sample in the gas flow are then vaporized by the heat transferred to the spray droplets in the desolvation chamber and leave the tube as a mixture of the compounds of interest and hot gas (120ºC).[4] They are subsequently carried along a corona discharge electrode where ionization occurs.
The ionization can either be carried out in positive or negative ionization mode. In the positive mode, the relative proton affinities of the reactant ions and the gaseous analyte molecules allow either proton transfer or adduction of reactant gas ions to produce the ions of the molecular species. [4] In the negative mode, however, the ions are produced by either proton abstraction or adduct formation.
In most cases, the evaporated mobile phase acts as the ionization gas and reactant ions are formed because of the effect of the corona discharge on the nebulized solvent. Generally, the primary ions formed by the corona discharge are ions such as a positively charged nitrogen or oxygen radical which can then form secondary reactant gas ions though collision with vaporized solvent molecules.[5]
In a major distinction from chemical ionization, the electrons needed for the primary ionization are not produced by a heated filament, as a heated filament cannot be used under atmospheric pressure conditions. Instead, the ionization must occur using either corona discharges or β- particle emitters, which are both electron sources capable of handling the presence of corrosive or oxidizing gases.[4]
Advantages
Ionization of the substrate is very efficient as it occurs at atmospheric pressure, and thus has a high collision frequency. Additionally, APCI considerably reduces the thermal decomposition of the analyte because of the rapid desolvation and vaporization of the droplets in the initial stages of the ionization. [4] This combination of factors most typically results in the production of ions of the molecular species with fewer fragmentations than many other ionization methods, making it a soft ionization method.[6]
Another advantage to using APCI over other ionization methods is that it allows for the high flow rates typical of standard bore HPLC (0.2-2.0mL/min) to be used directly, often without diverting the larger fraction of volume to waste. Additionally, APCI can often be performed in a modified ESI source. The ionization occurs in the gas phase, unlike ESI, where the ionization occurs in the liquid phase. A potential advantage of APCI is that it is possible to use a nonpolar solvent as a mobile phase solution, instead of a polar solvent, because the solvent and molecules of interest are converted to a gaseous state before reaching the corona discharge pin.
See also
References
- ↑ Carroll, D. I.; Dzidic, I.; Stillwell, R. N.; Horning, M. G.; Horning, E. C. (1974). "Subpicogram detection system for gas phase analysis based upon atmospheric pressure ionization (API) mass spectrometry". Analytical Chemistry. 46 (6): 706–710. doi:10.1021/ac60342a009. ISSN 0003-2700.
- ↑ J. R. Chapman (28 September 1995). Practical Organic Mass Spectrometry: A Guide for Chemical and Biochemical Analysis. John Wiley & Sons. ISBN 978-0-471-95831-4.
- ↑ Bruins, A. P. (1991). "Mass spectrometry with ion sources operating at atmospheric pressure". Mass Spectrometry Reviews. 10 (1): 53–77. doi:10.1002/mas.1280100104. ISSN 0277-7037.
- 1 2 3 4 5 Edmond de Hoffmann; Vincent Stroobant (22 October 2007). Mass Spectrometry: Principles and Applications. Wiley. ISBN 978-0-470-51213-5.
- ↑ Gates, Paul. University of Bristol, Department of Chemistry, "Atmospheric Pressure Chemical Ionization." Last modified 2004. Accessed November 22, 2013. "Archived copy". Archived from the original on 2013-11-26. Retrieved 2013-12-06..
- ↑ Zaikin, Vladimir; Halket, John (2006). "Review: Derivatization in mass spectrometry8. Soft ionization mass spectrometry of small molecules". European Journal of Mass Spectrometry. 12 (1): 79–115. doi:10.1255/ejms.798. ISSN 1356-1049. PMID 16723751.