Aristolochic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
8-Methoxy-6-nitrophenanthro[3,4-d][1,3]dioxole-5-carboxylic acid | |
| Other names
Aristinic acid; Aristolochia yellow; Aristolochic acid A; Aristolochin;Aristolochine; Descresept; Tardolyt;TR 1736 | |
| Identifiers | |
| 313-67-7 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL93353 |
| ChemSpider | 2149 |
| ECHA InfoCard | 100.005.673 |
| KEGG | C08469 |
| PubChem | 2236 |
| |
| |
| Properties | |
| C17H11NO7 | |
| Molar mass | 341.28 g·mol−1 |
| Appearance | yellow powder |
| Melting point | 260 to 265 °C (500 to 509 °F; 533 to 538 K) |
| Slightly soluble | |
| Hazards | |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Aristolochic acids (English /əˌrɪstəˈloʊkᵻk/) are a family of carcinogenic, mutagenic, and nephrotoxic compounds commonly found in the birthwort (Aristolochiaceae) family of plants. Aristolochic acid (AA) I is the most abundant one.[1] The Aristolochiaceae family includes the Aristolochia genus and the Asarum (wild ginger) genus, which are commonly used in Chinese herbal medicine.[2][3] Although these compounds are widely associated with kidney problems and urothelial cancers, the use of AA-containing plants for medicinal purposes has a long history. Nevertheless, the FDA has issued warnings regarding consumption of AA-containing supplements.
History
Early medical uses
Aristolochic acid first appeared in Chinese medicine in the fifth century AD, but the birthwort plants in which it is found are mentioned in ancient Greek and Roman medical texts dating back even earlier. In these ancient times, it was used to treat kidney and urinary problems, as well as gout, snakebites, and a variety of other ailments. It was also considered to be an effective contraceptive. In many of these cases, birthwort plants, and the aristolochic acids they contain, were just some of the many ingredients used to create ointments or salves. In the early first century, in Roman texts, aristolochic acids are first mentioned as a component of frequently ingested medicines to treat things such as asthma, hiccups, spasms, pains, and expulsion of afterbirth.[4]
Discovery of toxicity
Aristolochic acid poisoning was first diagnosed at a clinic in Brussels, Belgium, when cases of nephritis leading to rapid kidney failure were seen in a group of women who had all taken the same weight-loss supplement, Aristolochia fangchi, which contained aristolochic acid. This nephritis was termed “Chinese herbs nephropathy” (CHN) due to the origin of the weight-loss supplement.[5] A similar condition previously known as Balkan endemic nephropathy (BEN), first characterized in the 1950s in southeastern Europe, was later discovered to be also the result of aristolochic acid (AA) consumption. BEN is more slowly progressive than the nephritis that is seen in CHN, but is likely caused by low-level AA exposure, possibly from contamination of wheat flour seeds by a plant of the birthwort family, Aristolochia clematitis.[6] CHN and BEN fall under the umbrella of what is now known as aristolochic acid nephropathy, the prevalent symptom of AA poisoning.[5]
Biosynthesis
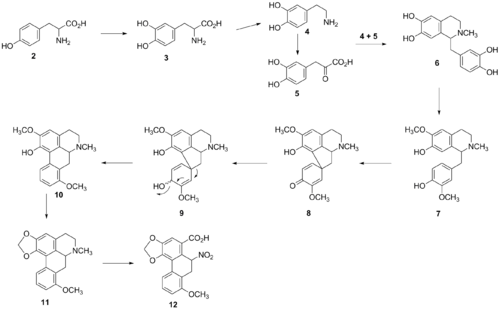
The aristolochic acids are among a group of substituted 10-nitro-1-phenantropic acids, biogenetically derived from benzylisoquinoline precursors, which in turn originate from tyrosine.[7] The biosynthesis of aristolochic acid, therefore, begins at tyrosine (2), which is converted to L-DOPA (3). DOPA is subsequently converted into dopamine (4) and further converstion of dopamine into 3,4-dihydroxyphenylacetaldehyde (5) (or 4-hydroxyphenylacetaldehyde to yield reticuline), results in the formation of norlaudanosoline (6) via a spontaneous Pictet-Spengler condensation of the two molecules.[8] Evidence of these precursors was confirmed by 14C experiments where labeled compounds were administered to Aristolochia sipho and aristolochic acid was successfully extracted. Modification of norlaudanosoline is continued with a methylation of two alcohol groups to give orientaline (7). Orientaline undergoes a cyclization event to give the addition of the 5-membered ring into orientalinol (8), subsequently undergoing a methyl shift and aromatization event (9) to yield prestephanine (10).[9] The final steps are the formation of stephanine (11) and the addition of a carboxylate to yield aristolochic acid (12). Comer et al. originally proposed a postulated pathway from norlaudanosoline to aristolochic acid in 1968 very similar to the described pathway, but Sharma et al. confirmed the presence of the intermediate compounds via DL-[3-14C] tyrosine trapping experiments in Aristolochia bracteata in 1982. After the general backbone of aristolochic acid is synthesized, postsynthetic modifications are performed; for example, aromatization of one of the rings yields aristolochic acid I.
Symptoms and diagnosis
Exposure to aristolochic acid is associated with a high incidence of uroepithelial tumorigenesis,[10] and is linked to urothelial cancer.[11][12] Since aristolochic acid is a mutagen, it does damage over time. Patients are often first diagnosed with aristolochic acid nephropathy (AAN), which is a rapidly progressive nephropathy and puts them at risk for renal failure and urothelial cancer. However, urothelial cancer is only observed long after consumption. One study estimated, on average, detectable cancer develops ten years from the start of daily aristolochic acid consumption.[5]
A patient thought to have AAN can be confirmed through phytochemical analysis of plant products consumed and detection of aristolactam DNA adducts in the renal cells. (Aristolochic acid is metabolised into aristolactam.) Additionally, mutated proteins in renal cancers as a result of transversion of A:T pairings to T:A are characteristically seen in aristolochic acid-induced mutations. In some cases, early detection resulting in cessation of aristolochia-product consumption can lead to reverse of the kidney damage.[6][13]
Pharmacology
ADME
Once orally ingested, aristolochic acid I is absorbed through the gastrointestinal tract into the blood stream.[6] It is distributed throughout the body via the blood stream.[6]
Aristolochic acids are metabolized by oxidation and reduction pathways, or phase I metabolism. Reduction of aristolochic acid I produces aristolactam I which has been observed in the urine. Further processing of aristolactam I by O-demethylation results in aristolactam Ia, the primary metabolite.[6][14] Additionally, nitroreduction results in an N-acylnitrenium ion, which can form DNA-base adducts, thus giving aristolochic acid I its mutagenic properties.[5][6][14]
Aristolactam I adducts that are bound to DNA are extremely stable; they have been detected in patient biopsy samples taken 20 years after exposure to plants containing aristolochic acid.[15]
Excretion of aristolochic acids and their metabolites is through the urine.[6]
Mechanism of action
The exact mechanism of action of aristolochic acid is not known, especially in regards to nephropathy. The carcinogenic effects of aristolochic acids are thought to be a result of mutation of the tumor suppressor gene TP53, which seems to be unique to aristolochic acid-associated carcinogenesis.[13] Nephropathy caused by aristolochic acid consumption is not mechanistically understood, but DNA adducts characteristic of aristolochic acid-induced mutations are found in the kidneys of AAN patients, indicating these might play a role.[13]
Regulation
In April 2001, the Food and Drug Administration issued a consumer health alert warning against consuming botanical products, sold as "traditional medicines" or as ingredients in dietary supplements, containing aristolochic acid.[16] The agency warned that consumption of aristolochic acid-containing products was associated with "permanent kidney damage, sometimes resulting in kidney failure that has required kidney dialysis or kidney transplantation. In addition, some patients have developed certain types of cancers, most often occurring in the urinary tract."[16]
In August 2013, two studies identified an aristolochic acid mutational signature in upper urinary tract cancer patients from Taiwan.[17][18] The carcinogenic effect is the most potent found thus far, exceeding the amount of mutations in smoking-induced lung cancer and UV-exposed melanoma. Exposure to aristolochic acid may also cause certain types of liver cancer.[17]
See also
References
- ↑ Wu, Tian-Shung; et al. (2005). "Chemical constituents and pharmacology of Aristolochia species". In Rahman, Atta-ur. Studies in Natural Products Chemistry: Bioactive Natural Products (Part L). Gulf Professional Publishing. p. 863. ISBN 978-0-444-52171-2.
- ↑ Heinrich M, Chan J, Wanke S, Neinhuis C, Simmonds MS (August 2009). "Local uses of Aristolochia species and content of nephrotoxic aristolochic acid 1 and 2--a global assessment based on bibliographic sources". J Ethnopharmacol. 125 (1): 108–44. doi:10.1016/j.jep.2009.05.028. PMID 19505558.
- ↑ Nolin, Thomas D. & Himmelfarb, Jonathan (2010). "Mechanisms of drug-induced nephrotoxicity". In Uetrecht, Jack. Adverse Drug Reactions. Springer. p. 123. ISBN 978-3-642-00662-3.
- ↑ Scarborough, John (2011). "Ancient Medicinal Use of Aristolochia: Birthwort's Tradition and Toxicity". Pharmacy in History. 53 (1): 3–21. Retrieved 3 May 2015.
- 1 2 3 4 Arlt, Volker; Stiborova, Marie; Schmeiser, Heinz (2002). "Aristolochic acid as a probable human cancer hazard in herbal remedies: a review". Mutagenesis. 17 (4): 265–277. doi:10.1093/mutage/17.4.265.
- 1 2 3 4 5 6 7 Lunn, Ruth; Jameson, C.W.; Jahnke, Gloria (2 Sep 2008). "Report on Carcinogens Background Document for Aristolochic Acids" (PDF). National Toxicology Program. Retrieved 3 May 2015.
- ↑ Comer, F.; Tiwari, H.P.; Spenser, I.D. (1969), "Biosynthesis of aristolochic acid", Canadian Journal of Chemistry, 47 (3): 481–487, doi:10.1139/v69-070
- ↑ Hoover, Larry K.; Moo-young, Murray; Legge, Raymond L. (1991), "Biotransformation of Dopamine to Norlaudanosoline by Aspergillus niger", Biotechnology and Bioengineering, 38 (9): 1029–1033, doi:10.1002/bit.260380911
- ↑ Sharma, Vidur; Jain, Sudha; Bhakuni, Dewan S.; Kapil, Randhir S. (1982), "Biosynthesis of aristolochic acid", Journal of the Chemical Society, Perkin Transactions 1, 1 (0): 1153–1155, doi:10.1039/p19820001153
- ↑ Ronco, Claudio et al., eds. (2008). Critical care nephrology. Elsevier Health Sciences. p. 1699. ISBN 978-1-4160-4252-5.
- ↑ Chen CH, Dickman KG, Moriya M, Zavadil J, Sidorenko VS, Edwards KL, Gnatenko DV, Wu L, Turesky RJ, Wu XR, Pu YS, Grollman AP (May 2012). "Aristolochic acid-associated urothelial cancer in Taiwan". Proc. Natl. Acad. Sci. U.S.A. 109 (21): 8241–6. doi:10.1073/pnas.1119920109. PMC 3361449
 . PMID 22493262.
. PMID 22493262. - ↑ Lai, M.-N.; Wang, S.-M.; Chen, P.-C.; Chen, Y.-Y.; Wang, J.-D. (2009). "Population-Based Case-Control Study of Chinese Herbal Products Containing Aristolochic Acid and Urinary Tract Cancer Risk". JNCI Journal of the National Cancer Institute. 102 (3): 179–186. doi:10.1093/jnci/djp467.
- 1 2 3 Go¨kmen, M. Refik; Cosyns, Jean-Pierre; Arlt, Volker M.; Stiborova, Marie; Phillips, David H.; Schmeiser, Heinz H.; Simmonds, Monique S.J.; Cook, Terence; Vanherweghem, Jean-Louis; Nortier, Joe¨lle L.; Lord, Graham M. (2013). "The Epidemiology, Diagnosis, and Management of Aristolochic Acid Nephropathy: A Narrative Review" (PDF). Annals of Internal Medicine. 158 (6): 469–477. doi:10.7326/0003-4819-158-6-201303190-00006. Retrieved 3 May 2015.
- 1 2 "Plants Containing Aristolochic Acid" (PDF). IARC Monographs-100A: 347–361. Retrieved 3 May 2015.
- ↑ Schmeiser; Nortier; Singh; da Costa; Sennesaei; Cassuto-Viguier; Ambrosetti; Rorive; Pozdzik; Phillips; Stiborova; Arlt (2014). "Exceptionally long-term persistence of DNA adducts formed by carcinogenic aristolochic acid I in renal tissue from patients with aristolochic acid nephropathy". International Journal of Cancer. 135 (2). doi:10.1002/ijc.28681.
- 1 2 FDA Warns Consumers to Discontinue Use of Botanical Products that Contain Aristolochic acid. April 11, 2001.
- 1 2 Poon, S. L.; Pang, S.-T.; McPherson, J. R.; Yu, W.; Huang, K. K.; Guan, P.; Weng, W.-H.; Siew, E. Y.; Liu, Y. (2013). "Genome-Wide Mutational Signatures of Aristolochic Acid and Its Application as a Screening Tool". Science Translational Medicine. 5 (197): 197ra101. doi:10.1126/scitranslmed.3006086. PMID 23926199.
- ↑ Hoang, M. L.; Chen, C.-H.; Sidorenko, V. S.; He, J.; Dickman, K. G.; Yun, B. H.; Moriya, M.; Niknafs, N.; Douville, C. (2013). "Mutational Signature of Aristolochic Acid Exposure as Revealed by Whole-Exome Sequencing". Science Translational Medicine. 5 (197): 197ra102. doi:10.1126/scitranslmed.3006200. PMID 23926200.
Further reading
- Aronson, J.K. (2008). "Aristolochicae". Meyler's side effects of herbal medicines. Elsevier. p. 55. ISBN 978-0-444-53269-5.
- Mills, Simon & Bone, Kerry (2005). "Aristolochic Acid Nephropathy". The essential guide to herbal safety. Elsevier Health Sciences. p. 15. ISBN 978-0-443-07171-3.
- Poon, Wing-Tat; Lai, Chi-Kong; Yan-Wo Chan, Albert (2007). "Aristolochic Acid Nephropathy: The Hong Kong Perspective". Hong Kong Journal of Nephrology. 9 (1): 7–14. doi:10.1016/s1561-5413(07)60003-9.
External links
| Wikimedia Commons has media related to Aristolochic acid. |
- Complete list of warnings from the US Food and Drug Administration
- FDA Concerned About Botanical Products, Including Dietary Supplements, Containing Aristolochic Acid May 2000.
- Plants Containing Aristolochic Acid
- Herbal medicines causing kidney failure, bladder cancer in India, Times of India, Mar 19, 2013
