Alkaline anion exchange membrane fuel cells
Alkaline anion exchange membrane fuel cells (AAEMFC) also known as hydroxide exchange membrane fuel cells (HEMFCs), anion-exchange membrane fuel cells (AEMFCs), or alkaline membrane fuel cells (AMFCs), are a type of alkaline fuel cells (AFCs). Alkaline fuel cells, one of the oldest type of fuel cell, operate between ambient temperature and 90 °C with an electrical efficiency higher than the other fuel cells such as proton exchange membrane fuel cells (PEMFC), solid oxide fuel cells and phosphoric acid fuel cells. Alkaline anion exchange membrane fuel cells (AAEMFCs) are functionally similar to AFCs, the difference being AAEMFCs employ a solid polymer electrolyte while AFCs use aqueous potassium hydroxide (KOH) as an electrolyte.
Science
Reactions
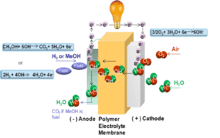
In an AAEMFC, the fuel, hydrogen or methanol, is supplied at the anode and oxygen through air, and water are supplied at cathode. Fuel is oxidized at anode and oxygen is reduced at cathode. At cathode, oxygen reduction produces hydroxides ions (OH−) that migrate through the electrolyte towards the anode. At anode, hydroxide ions react with the fuel to produce water and electrons. Electrons go through the circuit producing current.[1]
Electrochemical reactions when hydrogen is the fuel
At Anode: H2 + 2OH− → 2H2O + 2e−
At cathode: O2 + 2H2O + 4e− → 4OH−
Electrochemical reactions when methanol is the fuel
At anode: CH3OH + 6OH− → CO2 + 5H2O + 6e-
At cathode: 3/2O2 + 3H2O + 6e− → 6OH−
Comparison with traditional alkaline fuel cell
The alkaline fuel cell used by NASA in 1960s for Apollo and Space Shuttle program generated electricity at nearly 70% efficiency using aqueous solution of KOH as an electrolyte. In that situation, CO2 coming in through oxidant air stream and generated as by product from oxidation of methanol, if methanol is the fuel, reacts with electrolyte KOH forming CO32−/HCO3−. Unfortunately as a consequence, K2CO3 or KHCO3− precipitate on the electrodes. However, this effect has found to be mitigated by the removal of cationic counterions from the electrode, and carbonate formation has been found to be entirely reversible by several industrial and academic groups, most notably Varcoe. Low-cost CO2 systems have been developed using air as the oxidant source.[2] In alkaline anion exchange membrane fuel cell, aqueous KOH is replaced with a solid polymer electrolyte membrane, that can conduct hydroxide ions. This could overcome the problems of electrolyte leakage and carbonate precipitation, though still taking advantage of benefits of operating a fuel cell in an alkaline environment. In AAEMFCs, CO2 reacts with water forming H2CO3, which further dissociate to HCO3− and CO32−. The equilibrium concentration of CO32−/HCO3− is less than 0.07% and there is no precipitation on the electrodes in the absence of cations (K+, Na+).[3][4] The absence of cations is, however, difficult to achieve, as most membranes are conditioned to functional hydroxide or bicarbonate forms out of their initial, chemically stable halogen form, and may significantly impact fuel cell performance by both competitively adsorbing to active sites and exerting Helmholtz-layer effects.[5]
Advantages
The most significant advantage of AAEMFCs is that under alkaline conditions, oxygen reduction reaction (ORR) kinetics at the cathode are much more facile than in PEMFCs, potentially allowing use of inexpensive, non-noble metal catalysts such as silver or iron phthalocyanines for the cathode and nickel for the anode (fuel electrode). An alkaline medium accelerates oxidation of methanol making it an attractive fuel to be used. The large majority of membranes/ionomer that have been developed are fully hydrocarbon, allowing for much easier catalyst recycling and lower fuel crossover. Methanol has an advantage of easier storage and transportation and has higher volumetric energy density compared to hydrogen. Also, methanol crossover from anode to cathode is reduced in AAEMFCs compared to PEMFCs, due to the opposite direction of ion transport in the membrane, from cathode to anode. In addition, use of higher alcohols such as ethanol and propanol is possible in AAEMFCs, since anode potential in AAEMFCs is sufficient to oxidize C-C bonds present in alcohols.[6][7]
Challenges
The biggest challenge in developing AAEMFCs is the anion exchange membrane (AEM). A typical AEM is composed of a polymer backbone with tethered cationic ion-exchange groups to facilitate the movement of free OH− ions. This is the inverse of Nafion used for PEMFCs, where an anion is covalently attached to the polymer and protons hop from one site to another. The challenge is to fabricate AEM with high OH− ion conductivity and mechanical stability without chemical deterioration at elevated pH and temperatures. The main mechanisms of degradation are Hoffmann elimination when β-hydrogens are present and direct nucleophilic attack by OH− ion at the cationic site. One approach towards improving the chemical stability towards Hofmann elimination is to remove all β-hydrogens at the cationic site. All these degradation reactions limit the polymer backbone chemistries and the cations that can be incorporated for developing AEM.
Another challenge is achieving OH− ion conductivity comparable to H+ conductivity observed in PEMFCs. Since the diffusion coefficient of OH− ions is twice less than that of H+ (in bulk water), a higher concentration of OH− ions is needed to achieve similar results, which in turn needs higher ion exchange capacity of the polymer.[8] However, high ion exchange capacity leads to excessive swelling of polymer on hydration and concomitant loss of mechanical properties.
References
- ↑ Winter, M; Brodd, R. J. (2004). "What are batteries, fuel cells, and supercapacitors?". Chemical Reviews. 104 (10): 4245–4269. doi:10.1021/cr020730k. PMID 15669155.
- ↑ "US8628889 B2 - Operating method of anion-exchange membrane-type fuel cell".
- ↑ Adams, L. A.; Varcoe, J. R. (2008). Chem Sus Chem. 1: 79–81.
- ↑ Shen, P. K.; Xu, C. (2005). Adv. Fuel Cells: 149–179.
- ↑ Mills, J. N.; McCrum, I. T.; Janik, M. J. (2014). Phys. Chem. Chem. Phys.: 13699–13707.
- ↑ Varcoe, J. R.; Slade, R. C. T. (2005). "Prospects for Alkaline Anion-Exchange Membranes in Low Temperature Fuel Cells". Fuel Cells. 5: 187–200. doi:10.1002/fuce.200400045.
- ↑ Shen, P. K.; Xu, C. (2005). Adv. Fuel Cells: 149–179.
- ↑ Agel, E; Bouet, J.; Fauvarque, J.F (2001). "Characterization and use of anionic membranes for alkaline fuel cells". Journal of Power Sources. 101 (2): 267–274. doi:10.1016/s0378-7753(01)00759-5.