Acyloin condensation
| Acyloin condensation | |
|---|---|
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | acyloin-condensation |
| RSC ontology ID | RXNO:0000085 |
Acyloin condensation is a reductive coupling of two carboxylic esters using metallic sodium to yield an α-hydroxyketone, also known as an acyloin.[1][2][3]

The reaction is most successful when R is aliphatic and inert. The reaction is performed in aprotic solvents with a high boiling point, such as benzene and toluene. The use of protic solvents results in the Bouveault-Blanc reduction of the separate esters rather than condensation. Depending on ring size and steric properties, but independent from high dilution, the acyloin condensation of diesters favours intramolecular cyclisation over intermolecular polymerisation.

Mechanism
The mechanism consists of four steps:
- (1) Oxidative ionization of two sodium atoms on the double bond of two ester molecules.
- (2) Free radical coupling between two molecules of the homolytic ester derivative (A Wurtz type coupling). Alkoxy-eliminations in both sides occur, producing a 1,2-diketone.
- (3) Oxidative ionization of two sodium atoms on both diketone double bonds. The sodium enodiolate is formed.
- (4) Neutralization with water to form the enodiol, which tautomerizes to acyloin.[4]
Variations
Rühlmann-method
The method according to Rühlmann[5] employs trimethylchlorosilane as a trapping reagent; by this, competing reactions are efficiently subdued. Generally, yields increase considerably. The hydrolytic cleavage of the silylether gives the acyloin. To achieve a mild cleavage methanol can be used in several cases.
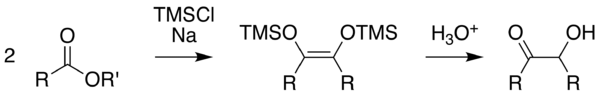
Usually toluene, dioxane, tetrahydrofuran or acyclic dialkylethers are employed as solvents. Advantageously also N-methyl-morpholine has been used. It allowed in some cases a successful reaction, in which otherwise the reaction failed in less polar media.
References
- ↑ Bouveault, L.; R. Loquin (1905). "Action du sodium sur les éthers des acides monobasiques à fonction simple de la série grasse". Compt. Rend. 140: 1593–1595.
- ↑ Finley, K. T. (1964). "The Acyloin Condensation as a Cyclization Method". Chemical Reviews. 64 (5): 573–589. doi:10.1021/cr60231a004.
- ↑ Bloomfield, J. J.; Owsley, D. C.; Nelke, J. M. Org. React. 1976, 23.
- ↑ Acyloin condensation
- ↑ Rühlmann K. (1971). "Die Umsetzung von Carbonsäureestern mit Natrium in Gegenwart von Trimethylchlorsilan" [Reaction of carboxylic acid esters with sodium in the presence of trimethylchlorosilane]. Synthesis. 1971 (5): 236–253. doi:10.1055/s-1971-21707.
