APICA (synthetic cannabinoid drug)
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
1345973-50-3 |
| PubChem (CID) | 71308155 |
| ChemSpider | 29341717 |
| Chemical and physical data | |
| Formula | C24H32N2O |
| Molar mass | 364.522 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
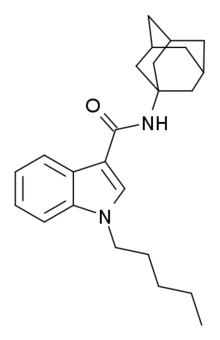
APICA (2NE1, SDB-001, N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide) is an indole based drug that acts as a potent agonist for the cannabinoid receptors.[1]
It had never previously been reported in the scientific or patent literature, and was first identified by laboratories in Japan in March 2012 as an ingredient in synthetic cannabis smoking blends, along with its indazole derivative APINACA (sold as "AKB48").[2]
Structurally it closely resembles cannabinoid compounds from patent WO 2003/035005 but with an indole core instead of indazole, and a simple pentyl chain on the indole 1-position.
Pharmacological testing determined APICA to have an IC50 of 175 nM at CB1, only slightly less potent than JWH-018 which had an IC50 of 169 nM, but over four times more tightly binding than APINACA, which had an IC50 of 824 nM.[3] The first published synthesis and pharmacological evaluation of APICA revealed that it acts as a full agonist at CB1 (EC50 = 34 nM) and CB2 receptors (EC50 = 29 nM).[4] Furthermore, APICA possesses cannabis-like effects in rats, and appears to be less potent than JWH-018 but more potent than THC.[4][5]
Legal Status
As of October 2015 APICA is a controlled substance in China.[6]
See also
References
- ↑ Carla Cannizzaro; Ginevra Malta; Antonina Argo; Anna Brancato; Gabriella Roda; Eleonora Casagni; Laura Fumagalli; Ermanno Valoti; Rino Froldi; Paolo Procaccianti; Veniero Gambaro (January 2016). "Behavioural And Pharmacological Characterization Of A Novel Cannabinomimetic Adamantane-Derived Indole, Apica, And Considerations On The Possible Misuse As A Psychotropic Spice Abuse, In C57bl/6 J Mice". Forensic Science International. doi:10.1016/j.forsciint.2015.12.035.
- ↑ Uchiyama, N.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. (2012). "Identification of two new-type synthetic cannabinoids, N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide (APICA) and N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide (APINACA), and detection of five synthetic cannabinoids, AM-1220, AM-2233, AM-1241, CB-13 (CRA-13), and AM-1248, as designer drugs in illegal products". Forensic Toxicology. 30 (2): 114–125. doi:10.1007/s11419-012-0136-7.
- ↑ Uchiyama, N.; Kawamura, M.; Kikura-Hanajiri, R.; Goda, Y. (2012). "URB-754: A new class of designer drug and 12 synthetic cannabinoids detected in illegal products". Forensic Science International. 227 (1–3): 21–32. doi:10.1016/j.forsciint.2012.08.047. PMID 23063179.
- 1 2 Banister, S. D.; Wilkinson, S. M.; Longworth, M.; Stuart, J.; Apetz, N.; English, K.; Brooker, L.; Goebel, C.; Hibbs, D. E.; Glass, M.; Connor, M.; McGregor, I. S.; Kassiou, M. (2013). "The synthesis and pharmacological evaluation of adamantane-derived indoles: Novel cannabimimetic drugs of abuse". ACS Chemical Neuroscience. 4 (7): 130403084729007. doi:10.1021/cn400035r. PMC 3715837
 . PMID 23551277.
. PMID 23551277. - ↑ Banister, S. D.; Stuart, J.; Kevin, R. C.; Edington, A.; Longworth, M.; Wilkinson, S. M.; Beinat, C.; Buchanan, A. S.; Hibbs, D. E.; Glass, M.; Connor, M.; McGregor, I. S.; Kassiou, M. (2015). "Effects of Bioisosteric Fluorine in Synthetic Cannabinoid Designer Drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135". ACS Chemical Neuroscience. 6: 150508124201002. doi:10.1021/acschemneuro.5b00107.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.