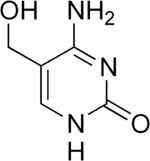
5-Hydroxymethylcytosine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
6-Amino-5-(hydroxymethyl)-1H-pyrimidin-2-one | |
| Identifiers | |
| 1123-95-1 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:76792 |
| ChemSpider | 63916 |
| PubChem | 70751 |
| |
| |
| Properties | |
| C5H7N3O2 | |
| Molar mass | 141.13 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
5-Hydroxymethylcytosine is a DNA pyrimidine nitrogen base derived from cytosine. It is potentially important in epigenetics, because the hydroxymethyl group on the cytosine can possibly switch a gene on and off. It was first seen in bacteriophages in 1952.[1][2] However, in 2009 it was found to be abundant in human and mouse brains,[3] as well as in embryonic stem cells.[4] In mammals, it can be generated by oxidation of 5-methylcytosine, a reaction mediated by the Tet family of enzymes. Its molecular formula is C5H7N3O2.[5]
Localization
Every mammalian cell seems to contain 5-Hydroxymethylcytosine, but the levels vary significantly depending on the cell type. The highest levels are found in neuronal cells of the central nervous system.[6][7][8] The amount of hydroxymethylcytosine increases with age, as shown in mouse hippocampus and cerebellum.[6][9]
Function
The exact function of this nitrogen base is still not fully elucidated, but it is thought that it may regulate gene expression or prompt DNA demethylation. This hypothesis is supported by the fact that artificial DNA that contains 5-hydroxymethylcytosines (5hmC) can be converted into unmodified cytosines once introduced into mammalian cells.[10] Moreover, 5hmC is highly enriched in primordial germ cells, where it apparently plays a role in global DNA demethylation.[11] Additionally, 5-Formylcytosine, an oxidation product of 5-Hydroxymethylcytosine and possible intermediate of an oxidative demethylation pathway was detected in DNA from embryonic stem cells,[12] although no significant amounts of these putative demethylation intermediates could be detected in mouse tissue.[8] 5-Hydroxymethylcytosine may be especially important in the central nervous system, as it is found in very high levels there.[8] Reduction in the 5-Hydroxymethylcytosine levels have been found associated with impaired self-renewal in embryonic stem cells.[13] 5-Hydroxymethylcytosine is also associated with labile, unstable nucleosomes which are frequently repositioned during cell differentiation.[14]
History
5-Hydroxymethylcytosine was observed by Skirmantas Kriaucionis, an associate at the Heintz lab, who was looking for levels of 5-methylcytosine in two different neuron types. He discovered a significant amount of an unknown substance instead, and after conducting several tests, identified it as being 5-hydroxymethylcytosine.[15]
The lab of L. Aravind used bioinformatic tools to predict that the Tet family of enzymes would likely oxidize 5-methylcytosine to 5-hydroxymethylcytosine.[16] This was demonstrated in vitro and in live human and mouse cells by scientists working in the labs of Anjana Rao and David R. Liu.
5-Hydroxymethylcytosine was originally observed in mammals in 1972 by R. Yura,[17] but this initial finding is dubious. Yura found 5-hmC present at extremely high levels in rat brain and liver, completely supplanting 5-methylcytosine. This contradicts all research conducted on mammalian DNA composition conducted before and since, including the Heintz and Rao papers, and another group was unable to reproduce Yura's result.[18]
With the discovery of 5-hydroxymethylcytosine some concerns have been raised regarding DNA methylation studies using the bisulfite sequencing technique.[19] 5-hydroxymethylcytosine has been shown to behave like its precursor, 5-methylcytosine, in bisulfite conversion experiments.[20] Therefore, bisulfite sequencing data may need to be revisited to verify whether the detected modified base is 5-methylcytosine or 5-hydroxymethylcytosine. In 2012 the lab of Chuan He discovered a method to solve the problems of 5-hydroxymethylcytosine being detected as 5-methylcytosine in normal bisulfite conversion experiments using the oxidative properties of the Tet-family of enzymes, this method has been termed TAB-seq.[21][22]
References
- ↑ Warren, RA (1980). "Modified bases in bacteriophage DNAs". Annu. Rev. Microbiol. 34: 137–158. doi:10.1146/annurev.mi.34.100180.001033. PMID 7002022.
- ↑ Wyatt, GR; Cohen, SS (December 1952). "A new pyrimidine base from bacteriophage nucleic acids". Nature. 170 (4338): 1072–1073. doi:10.1038/1701072a0. PMID 13013321.
- ↑ Kriaucionis, S; Heintz, N (May 2009). "The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain". Science. 324 (5929): 929–930. doi:10.1126/science.1169786. PMC 3263819
 . PMID 19372393.
. PMID 19372393. - ↑ Tahiliani M; et al. (May 2009). "Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1". Science. 324 (5929): 930–935. doi:10.1126/science.1170116. PMC 2715015
 . PMID 19372391.
. PMID 19372391. - ↑ 5-Hydroxymethylcytosine, nextbio.com
- 1 2 Münzel M; et al. (July 2010). "Quantification of the Sixth DNA Base Hydroxymethylcytosine in the Brain". Angew. Chem. Int. Ed. 49 (31): 5375–5377. doi:10.1002/anie.201002033.
- ↑ Szwagierczak A; et al. (October 2010). "Sensitive Enzymatic Quantification of 5-Hydroxymethylcytosine in Genomic DNA". Nucleic Acids Res. 38 (19): e181. doi:10.1093/nar/gkq684. PMC 2965258
 . PMID 20685817.
. PMID 20685817. - 1 2 3 Globisch D; et al. (December 2010). Croft, Anna Kristina, ed. "Tissue Distribution of 5-Hydroxymethylcytosine and Search for Active Demethylation Intermediates". PLoS ONE. 5 (12): e15367. doi:10.1371/journal.pone.0015367. PMC 3009720
 . PMID 21203455.
. PMID 21203455. - ↑ Song C-X; et al. (December 2010). "Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine". Nat. Biotechnol. 29 (1): 68–72. doi:10.1038/nbt.1732.
- ↑ Guo, Junjie U.; Su, Yijing; Zhong, Chun; Ming, Guo-li; Song, Hongjun (1 April 2011). "Hydroxylation of 5-Methylcytosine by TET1 Promotes Active DNA Demethylation in the Adult Brain". Cell. 145 (3): 423–434. doi:10.1016/j.cell.2011.03.022. PMC 3088758
 . PMID 21496894.
. PMID 21496894. - ↑ Hackett, JA; Sengupta, R; Zylicz, JJ; Murakami, K; Lee, C; Down, T; Surani, MA (2012-12-06). "Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine". Science. 339 (6118): 448–52. doi:10.1126/science.1229277. PMID 23223451.
- ↑ Pfaffeneder, Toni; Hackner, Benjamin; Truss, Matthias; Münzel, Martin; Müller, Markus; Deiml, Christian A.; Hagemeier, Christian; Carell, Thomas (30 June 2011). "The Discovery of 5-Formylcytosine in Embryonic Stem Cell DNA". Angew. Chem. Int. Ed. 50 (31): 7008–7012. doi:10.1002/anie.201103899. PMID 21721093.
- ↑ Freudenberg, JM; Ghosh, S; Lackford, BL; Yellaboina, S; Zheng, X; Li, R; Cuddapah, S; Wade, PA; Hu, G; Jothi, R (April 2012). "Acute depletion of Tet1-dependent 5-hydroxymethylcytosine levels impairs LIF/Stat3 signaling and results in loss of embryonic stem cell identity". Nucleic Acids Research. 40 (8): 3364–3377. doi:10.1093/nar/gkr1253. PMC 3333871
 . PMID 22210859.
. PMID 22210859. - ↑ Teif, Vladimir; Beshnova, Daria A.; Vainshtein, Yevhen; Marth, Caroline; Mallm, Jan-Philipp; Höfer, Thomas; Rippe, Karsten (8 May 2014). "Nucleosome repositioning links DNA (de)methylation and differential CTCF binding during stem cell development". Genome Research. 24 (8): 1285–1295. doi:10.1101/gr.164418.113. PMID 24812327.
- ↑ A, T, G, C and What?, popsci.com
- ↑ Iyer LM; et al. (June 2009). "Prediction of novel families of enzymes involved in oxidative and other complex modifications of bases in nucleic acids". Cell Cycle. 8 (11): 1698–1710. doi:10.4161/cc.8.11.8580. PMC 2995806
 . PMID 19411852.
. PMID 19411852. - ↑ Penn, NW; Suwalski, R; O'Riley, C; Bojanowski, K; Yura, R (February 1972). "The presence of 5-hydroxymethylcytosine in animal deoxyribonucleic acid". Biochem. J. 126 (4): 781–790. PMC 1178489
 . PMID 4538516.
. PMID 4538516. - ↑ Kothari, Rm; Shankar, V (May 1976). "5-Methylcytosine content in the vertebrate deoxyribonucleic acids: species specificity". Journal of Molecular Evolution. 7 (4): 325–329. doi:10.1007/BF01743628. ISSN 0022-2844. PMID 933178.
- ↑ 5 hydroxymethylcytosine analysis techniques
- ↑ Jin SG et al. (Jun 2010) "Examination of the specificity of DNA methylation profiling techniques towards 5-methylcytosine and 5-hydroxymethylcytosine." Nucleic Acids Res. 2010 Jun 1;38(11):e125
- ↑ Yu M, Hon GC, Szulwach KE, Song CX, Zhang L, Kim A, Li XK, Dai Q, Shen Y, Park B, Min JH, Jin P, Ren B, He C (June 2012). "Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome.". Cell. 149 (6): 1368–1380. doi:10.1016/j.cell.2012.04.027.
- ↑ Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, Li Y, Chen CH, Zhang W, Jian X, Wang J, Zhang L, Looney TJ, Zhang B, Godley LA, Hicks LM, Lahn BT, Jin P, He C (2011). "Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine". Nat. Biotechnol. 29 (1): 68–72. doi:10.1038/nbt.1732.