5-Hydroxytryptophan
.svg.png) | |
 | |
| Names | |
|---|---|
| IUPAC name
2-amino-3-(5-hydroxy-1H-indol-3-yl)propanoic acid | |
| Identifiers | |
| 4350-09-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:17780 |
| ChEMBL | ChEMBL350221 |
| ChemSpider | 388413 |
| ECHA InfoCard | 100.022.193 |
| 4671 | |
| KEGG | D07339 |
| MeSH | 5-Hydroxytryptophan |
| PubChem | 144 |
| UNII | C1LJO185Q9 |
| |
| |
| Properties | |
| C11H12N2O3 | |
| Molar mass | 220.23 g·mol−1 |
| Density | 1.484 g/mL |
| Melting point | 298 to 300 °C (568 to 572 °F; 571 to 573 K) |
| Boiling point | 520.6 °C (969.1 °F; 793.8 K) |
| Pharmacology | |
| N06AX01 (WHO) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
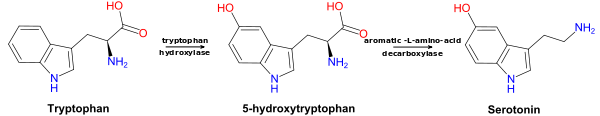
5-Hydroxytryptophan (5-HTP), also known as oxitriptan (INN), is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitters serotonin and melatonin from tryptophan.
Uses
5-HTP is sold over the counter in the United States, Canada, and the United Kingdom as a dietary supplement for use as an antidepressant, appetite suppressant, and sleep aid. It is also marketed in many European countries for the indication of major depression under the trade names Cincofarm, Levothym, Levotonine, Oxyfan, Telesol, Tript-OH, and Triptum.[1]
A 2002 review by the Cochrane Collaboration concluded that although the data evaluated suggests that 5-HTP is more effective than placebo in the treatment of depression, the evidence was insufficient to be conclusive due to a severe lack of high quality research.[2] More and larger studies are needed to determine if 5-HTP is truly effective in treating depression.[3]
5-HTP is sometimes taken by people coming down from MDMA to relieve post-MDMA dysphoria.[4] As 5-HTP is a necessary precursor for the brain to produce more serotonin, and MDMA use depletes a person's natural serotonin levels, it is believed that taking 5-HTP after consuming MDMA will speed up serotonin production. DanceSafe claims that the anecdotal evidence is widespread and that the theory is physiologically reasonable.[5] A research conducted by Wang et al. in 2007 suggested a recovery, when MDMA-induced depletions of 5-HT were restored in rats after administering 5-HTP.[6]
Side effects
Potential side effects of 5-HTP include heartburn, stomach pain, nausea, vomiting, diarrhea, drowsiness, sexual problems, vivid dreams or nightmares, and muscle problems.[7] Because 5-HTP has not been thoroughly studied in a clinical setting, possible side effects and interactions with other drugs are not well known.
Interactions
When combined with antidepressants of the MAOI or SSRI class, high dose 5-HTP can cause acute serotonin syndrome in rats.[8][9] In humans 5-HTP has never been clinically associated with serotonin syndrome, although 5-HTP can precipitate mania when added to a MAOI.[10] Due to the rate-limiting nature of the decarboxylase enzyme which converts 5-HTP into serotonin, the risk of serotonin syndrome with monoamine oxidase inhibitors is thought to be quite low unless both MAOIs and 5-HTP are taken in large quantities.
When combined with carbidopa (as a treatment for symptoms of Parkinson's disease), 5-HTP causes nausea and vomiting; however this can be alleviated via administration of granisetron.[11] As mentioned above under pharmacology, cases of scleroderma-like illness have been reported in patients using carbidopa and 5-HTP.[12]
Oral 5-HTP results in an increase in urinary 5-HIAA, a serotonin metabolite, indicating that 5-HTP is peripherally metabolized to serotonin, which is then metabolized. This might cause a false positive test in tests looking for carcinoid syndrome.[13][14] Due to the conversion of 5-HTP into serotonin by the liver, there may be a significant risk of heart valve disease from serotonin's effect on the heart.[15][16]
It has been suggested that 5-HTP may cause eosinophilia-myalgia syndrome (EMS), a serious condition which results in extreme muscle tenderness, myalgia, and blood abnormalities. However, there is evidence to show that EMS was likely caused by a contaminant in certain 5-HTP supplements.[17]
Metabolism
5-HTP is decarboxylated to serotonin (5-hydroxytryptamine or 5-HT) by the enzyme aromatic-L-amino-acid decarboxylase with the help of vitamin B6.[18] This reaction occurs both in nervous tissue and in the liver.[19] 5-HTP crosses the blood–brain barrier,[20] while 5-HT does not. Excess 5-HTP, especially when administered with vitamin B6, is thought to be metabolized and excreted.[21][22]
| ||||||||||||||||||||
Pharmacology
The psychoactive action of 5-HTP is derived from its increase in production of serotonin in central nervous system tissue.[23]
Research shows that co-administration with carbidopa greatly increases plasma 5-HTP levels.[24] However, several studies have reported that 5-HTP is effective even without a peripheral decarboxylase inhibitor (e.g. carbidopa).[25] Other studies have indicated the risk of a scleroderma-like condition resulting from the combination of 5-HTP and carbidopa.[26]
Dietary sources
Though 5-HTP is found in food only in insignificant quantities, it is a chemical involved intermediately in the metabolism of tryptophan, an amino acid found in milk, meat, potatoes, pumpkin, and various greens.[27]
The seeds of the Griffonia simplicifolia, a climbing shrub native to West Africa and Central Africa, are used as an herbal supplement for their 5-hydroxytryptophan (5-HTP) content.[28][29][30] In one 2010 trial, Griffonia simplicifolia extract appeared to increase satiety in overweight women.[31]
See also
References
- ↑ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- ↑ Shaw K, Turner J, Del Mar C (2002). Shaw KA, ed. "Tryptophan and 5-hydroxytryptophan for depression". Cochrane Database of Systematic Reviews (1): CD003198. doi:10.1002/14651858.CD003198. PMID 11869656.
- ↑ 5-Hydroxytryptophan (5-HTP) University of Maryland Medical Center. 2011. Accessed: 9 January 2012.
- ↑ Wang, X.; Baumann, M. H.; Dersch, C. M.; Rothman, R. B. (2007-08-10). "Restoration of 3,4-methylenedioxymethamphetamine-induced 5-HT depletion by the administration of l-5-hydroxytryptophan". Neuroscience. 148 (1): 212–220. doi:10.1016/j.neuroscience.2007.05.024. PMID 17629409.
- ↑ "Ecstasy and Depression". DanceSafe.Org. nd. Retrieved 21 September 2015.
- ↑ "Restoration of 3,4-methylenedioxymethamphetamine-induced 5-HT depletion by the administration of L-5-hydroxytryptophan". Neuroscience. 148 (1): 212–220. 12 July 2007. doi:10.1016/j.neuroscience.2007.05.024. PMID 17629409.
- ↑ "5-HTP". U.S. National Library of Medicine. Retrieved 7 June 2015.
- ↑ Ma Z, Zhang G, Jenney C, Krishnamoorthy S, Tao R (July 2008). "Characterization of serotonin-toxicity syndrome (toxidrome) elicited by 5-hydroxy-l-tryptophan in clorgyline-pretreated rats". Eur J Pharmacol. 588 (2–3): 198–206. doi:10.1016/j.ejphar.2008.04.004. PMID 18499101.
- ↑ Izumi T, Iwamoto N, Kitaichi Y, Kato A, Inoue T, Koyama T (2006). "Effects of co-administration of a selective serotonin reuptake inhibitor and monoamine oxidase inhibitors on 5-HT-related behavior in rats". Eur J Pharmacol. 532 (3): 258–264. doi:10.1016/j.ejphar.2005.12.075. PMID 16488409.
- ↑ Pardo, JV (2012). "Mania following addition of hydroxytryptophan to monoamine oxidase inhibitor.". General hospital psychiatry. 34 (1): 102.e13–4. doi:10.1016/j.genhosppsych.2011.08.014. PMID 21963353.
- ↑ Jacobs G, Kamerling I, de Kam M, et al. (Nov 2008). "Enhanced tolerability of the 5-hydroxytryptophane challenge test combined with granisetron". J Psychopharmacol. (Oxford). 24 (1): 65–72. doi:10.1177/0269881108094299. PMID 18719048.
- ↑ "Carbidopa/Levodopa". Truestarhealth.com. Archived from the original on 8 January 2014. Retrieved 2014-01-09.
- ↑ Joy T, Walsh G, Tokmakejian S, Van Uum SH (January 2008). "Increase of urinary 5-hydroxyindoleacetic acid excretion but not serum chromogranin A following over-the-counter 5-hydroxytryptophan intake". Can. J. Gastroenterol. 22 (1): 49–53. PMC 2659120
 . PMID 18209781.
. PMID 18209781. - ↑ Hallin, ML; Mahmoud, K; Viswanath, A; Gama, R (January 2013). "'Sweet Dreams', 'Happy Days' and elevated 24-h urine 5-hydroxyindoleacetic acid excretion.". Annals of Clinical Biochemistry. 50 (Pt 1): 80–2. doi:10.1258/acb.2012.012041. PMID 23086978.
- ↑ Gustafsson BI, Tømmerås K, Nordrum I, Loennechen JP, Brunsvik A, Solligård E, Fossmark R, Bakke I, Syversen U, Waldum H (Mar 2005). "Long-term serotonin administration induces heart valve disease in rats". Circulation. 111 (12): 1517–22. doi:10.1161/01.CIR.0000159356.42064.48. PMID 15781732.
- ↑ Xu J, Jian B, Chu R, Lu Z, Li Q, Dunlop J, Rosenzweig-Lipson S, McGonigle P, Levy RJ, Liang B (Dec 2002). "Serotonin mechanisms in heart valve disease II: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells". The American Journal of Pathology. 161 (6): 2209–18. doi:10.1016/S0002-9440(10)64497-5. PMC 1850896
 . PMID 12466135.
. PMID 12466135. - ↑ Michelson, D; Page, SW; Casey, R; Trucksess, MW; Love, LA; Milstien, S; Wilson, C; Massaquoi, SG; Crofford, LJ; Hallett, M (December 1994). "An eosinophilia-myalgia syndrome related disorder associated with exposure to L-5-hydroxytryptophan". The Journal of rheumatology. 21 (12): 2261–5. PMID 7699627.
- ↑ Rahman MK, Nagatsu T, Sakurai T, Hori S, Abe M, Matsuda M (1982). "Effect of pyridoxal phosphate deficiency on aromatic L-amino acid decarboxylase activity with L-DOPA and L-5-hydroxytryptophan as substrates in rats". Jpn. J. Pharmacol. 32 (5): 803–11. doi:10.1254/jjp.32.803. PMID 6983619.
- ↑ Bouchard, S; Bousquet, C; Roberge, AG (1981). "Characteristics of dihydroxyphenylalanine/5-hydroxytryptophan decarboxylase activity in brain and liver of cat". Journal of Neurochemistry. 37 (3): 781–7. doi:10.1111/j.1471-4159.1982.tb12555.x. PMID 6974228.
- ↑ Nakatani Y, Sato-Suzuki I, Tsujino N, et al. (May 2008). "Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat". Eur. J. Neurosci. 27: 2466–72. doi:10.1111/j.1460-9568.2008.06201.x. PMID 18445233.
- ↑ Bouchard S, Roberge AG (1979). "Biochemical properties and kinetic parameters of dihydroxyphenylalanine--5-hydroxytryptophan decarboxylase in brain, liver, and adrenals of cat". Can. J. Biochem. 57 (7): 1014–8. doi:10.1139/o79-126. PMID 39668.
- ↑ Amamoto T, Sarai K (1976). "On the tryptophan-serotonin metabolism in manic-depressive disorders. Changes in plasma 5-HT and 5-HIAA levels and urinary 5-HIAA excretion following oral loading of L-5HTP in patients with depression". Hiroshima J. Med. Sci. 25 (2–3): 135–40. PMID 1088369.
- ↑ "5-HTP: Uses, Side Effects, Interactions and Warnings - WebMD". Archived from the original on 16 November 2009. Retrieved 2009-10-05.
- ↑ Magnussen I, Jensen TS, Rand JH, Van Woert MH (1981). "Plasma accumulation of metabolism of orally administered single dose L-5-hydroxytryptophan in man". Acta pharmacologica et toxicologica. 49 (3): 184–9. doi:10.1111/j.1600-0773.1981.tb00890.x. PMID 6175178.
- ↑ Birdsall TC (1998). "5-Hydroxytryptophan: a clinically-effective serotonin precursor". Alternative Medicine Review. 3 (4): 271–80. PMID 9727088.
- ↑ Sternberg EM, Van Woert MH, Young SN, et al. (1980). "Development of a scleroderma-like illness during therapy with L-5-hydroxytryptophan and carbidopa". N. Engl. J. Med. 303 (14): 782–7. doi:10.1056/NEJM198010023031403. PMID 6997735.
- ↑ "5-Hydroxytryptophan". University of Maryland Medical Center. Archived from the original on 6 January 2010. Retrieved 21 January 2010.
- ↑ "5-Hydroxytryptophan (5-HTP)". A.D.A.M., Inc. University of Maryland Medical Center. Animated Dissection of Anatomy for Medicine, Inc. (A.D.A.M., Inc.) provided health and benefits information and technology to healthcare organizations, employers, consumers, and educational institutions
- ↑ Emanuele, E; Bertona, M; Minoretti, P; Geroldi, D (2010). "An open-label trial of L-5-hydroxytryptophan in subjects with romantic stress". Neuro endocrinology letters. 31 (5): 663–6. PMID 21178946.
- ↑ "5-hydroxy-L-tryptophan", National Center for Biotechnology Information, PubChem Compound Database, September 2004CID=439280
- ↑ Rondanelli M; Opizzi A; Faliva M; Bucci M; Perna S. (Mar 2012). "Relationship between the absorption of 5-hydroxytryptophan from an integrated diet, by means of Griffonia simplicifolia extract, and the effect on satiety in overweight females after oral spray administration". Eat Weight Disord. 17: e22–8. doi:10.3275/8165. PMID 22142813.
.svg.png)
