3-Pentanone
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Pentan-3-one | |
| Other names
Diethyl ketone, diethylketone, 3-pentanone, dimethyl acetone, propione, metacetone, methacetone, ethyl ketone | |
| Identifiers | |
| 96-22-0 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | DEK |
| ChEBI | CHEBI:87755 |
| ChEMBL | ChEMBL45315 |
| ChemSpider | 7016 |
| ECHA InfoCard | 100.002.265 |
| UNII | 9SLZ98M9NK |
| |
| |
| Properties | |
| C5H10O | |
| Molar mass | 86.13 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Odor | Acetone-like |
| Density | 0.815 g/cm3 |
| Melting point | −39 °C (−38 °F; 234 K) |
| Boiling point | 100 to 102 °C (212 to 216 °F; 373 to 375 K) |
| Solubility in other solvents | water: 50 g/L (20 °C),[2] organic solvents |
| Vapor pressure | 35 mmHg (25°C)[1] |
| Hazards | |
| Flash point | 12.78 °C (55.00 °F; 285.93 K) |
| 425 °C (797 °F; 698 K) | |
| Explosive limits | 1.6%-6.4%[1] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[1] |
| REL (Recommended) |
TWA 200 ppm (705 mg/m3)[1] |
| IDLH (Immediate danger) |
N.D.[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
3-Pentanone (also known as diethyl ketone) is a simple, symmetrical dialkyl ketone. It is a colorless liquid ketone with an odor like that of acetone. It is soluble in about 25 parts water, but miscible with organic solvents. It is mainly used as a solvent in paint and a precursor to vitamin E.[3] Two related and more important ketones are 2-pentanone and methyl isopropyl ketone.
Syntheses
Ketonic decarboxylation route
3-Pentanone is produced by ketonic decarboxylation of propanoic acid using metal oxide catalysts:
- 2 CH3CH2CO2H → (CH3CH2)2CO + CO2 + H2O
in the laboratory, the reaction cain be conducted in a tube furnace.[4]
Carbonylation route
It can also be prepared by combining ethylene, CO, and H2.[3] When the reaction is catalyzed by dicobalt octacarbonyl, waster can be used as a source of hydrogen. A proposed intermediate is the ethylene-propionyl species [CH3C(O)Co(CO)3(ethylene)] which undergoes a migratory insertion to form [CH3COCH2CH2Co(CO)3]. The required hydrogen arises from the water shift reaction. For details, see[5]
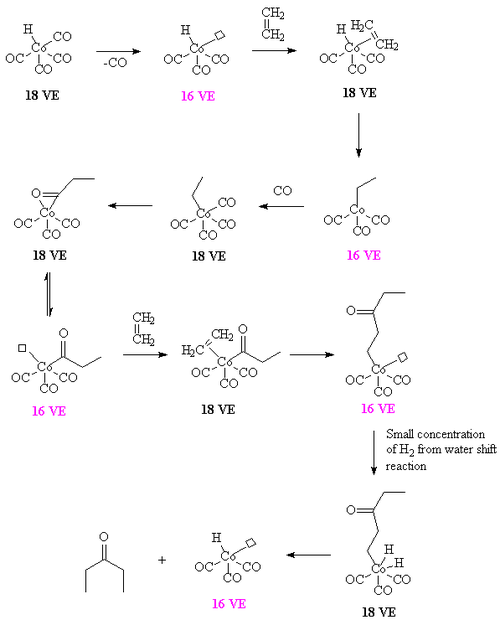
If the water shift reaction is not operative, the reaction affords a polymer containing alternating carbon monoxide and ethylene units. Such aliphatic polyketones are more conventionally prepared using palladium catalysts.[6]
Safety
The TLV value for 3-pentanone is 200 ppm (705 mg/m3).[3] 3-pentanone can be hazardous if it comes in contact with the skin or eyes, and can cause irritation of the skin and redness, watering, and itching of the eyes. This chemical can also cause nervous system or organ damage if ingested. Although considered stable, 3-pentanone is extremely flammable if exposed to flame, sparks, or another source of heat. For safety, it should be stored in a flammable materials cabinet away from heat or sources of ignition, preferably in a cool, well-ventilated area.[2]
References
- 1 2 3 4 5 6 "NIOSH Pocket Guide to Chemical Hazards #0212". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 Chemicals & Laboratory Equipment, Material Safety Data Sheet for 3-pentanone, ScienceLab.com, updated 11/06/2008
- 1 2 3 Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, 2002 by Wiley-VCH, Wienheim. doi:10.1002/14356007.a15_077
- ↑ Furniss, Brian; Hannaford, Antony; Smith, Peter & Tatchell, Austin (1996). Vogel's Textbook of Practical Organic Chemistry (5th ed.). London: Longman Science & Technical. p. 613. ISBN 9780582462366.
- ↑ Murata K.; Matsuda A. (1981). "Application of Homogeneous Water-Gas Shift Reaction III Further Study of the Hydrocarbonylation – A highly Selective Formation of Diethyl Keton from Ethene, CO and H2O". Bulletin of the Chemical Society of Japan. 54 (7): 2089–2092. doi:10.1246/bcsj.54.2089.
- ↑ J. Liu; B.T. Heaton; J.A. Iggo & R. Whyman (2004). "The Complete Delineation of the Initiation, Propagation, and Termination Steps of the Carbomethoxy Cycle for the Carboalkoxylation of Ethene by Pd–Diphosphane Catalysts". Angew. Chem. Int. Ed. 43: 90–94. doi:10.1002/anie.200352369.